Compute the percentage void space per unit volume of unit cell in zinc fluoride structure.
1. 15.03%
2. 22.18%
3. 18.23%
4. 25.07%
8 : 8 co-ordination of CsCl is found to change into 6 : 6 co-ordination on:
1. Applying pressure.
2. Increasing temperature.
3. Both 1 and 2.
4. None of these.
The elements of symmetry in a crystal are:-
1. Plane of symmetry
2. Axis of symmetry
3. Centre of symmetry
4. All of the above
For a solid with the following structure, the co-ordination number of the point B is:
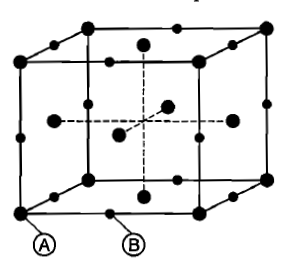
1. 3
2. 4
3. 5
4. 6
TiO2 is a well-known example of:
1. Triclinic system.
2. Tetragonal system.
3. Monoclinic system.
4. None of the above.
How many octahedral and tetrahedral holes are present per unit cell in a face centred cubic arrangement of atoms?
1. 8,4
2. 1,2
3. 4,8
4. 2 ,1
The density of KCl is 1.9893 g cm-3 and the length of a side unit cell is 6.29082 Å as determined by X-ray diffraction. The value of Avogadro’s number
calculated from this data is:-
1. 6.017 x 1023
2. 6.023 x 1022
3. 7.03 x 1023
4. 6.01 x 1019
Frenkel defect is noticed in:
1. AgBr
2. ZnS
3. Agl
4. All of the above
Close packing is maximum in the crystal lattice of:
1. simple cubic
2. face centred
3. body centred
4. none of these
A spinel is an important class of oxides consisting of two types of metal ions with the oxide ions arranged in ccp layers. The normal spinel has 1/8th of the tetrahedral void occupied by one type of metal and one half of the octahedral voids occupied by another type of metal ions. Such a spinel is formed by Zn2+, Al3+ and O2- with Zn2+ in tetrahedral void. Give the simplest formula of the spinel.
1. ZnAl2O4
2. ZnAl2O3
3. ZnAlO
4. None of these






