In adding 1 mL of solution of 10 % NaCl to 10 mL of gold solution in the presence of 0.25 g of starch, the coagulation is just prevented. Starch has a gold number equal to:
1. 0.25
2. 2.5
3. 250
4. 0.025
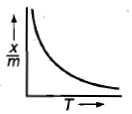
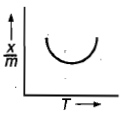
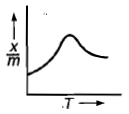
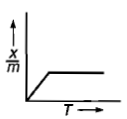
Which plot is the adsorption isobar for chemisorption where X is the amount of gas adsorbed on mass m (at constant pressure) at temperature T
1. 
2. 
3. 
4. 
What is a solution that isn't a lyophilic colloid?
1. Milk
2. Gum
3. Fog
4. Blood
Hardy-Schulze rule states that:
1. non-electrolytes have better coagulating action on colloids than electrolytes
2. sols are coagulated by effective ions whose charge is opposite to that of sol and the ions of higher charge are much more effective than the ions of lower charge
3. charge of the ions has no effect on the coagulation of a sol
4. sols are coagulated only by those ions whose charge is similar to that of the sol
An example of intrinsic colloid is:
1. As2S3 sol
2. Fe(OH)3 sol
3. egg albumin
4. Au sol
Tails of comets are visible due to:
1. Tyndall effect
2. Reflection
3. Brownian motion
4. None of the above
Bleeding is stoped by the application of ferric chloride. This is because :
1. the blood starts flowing in the opposite direction
2. the blood reacts and a solid is formed which seals the blood vessel
3. the blood is coagulated and the blood vessel are sealed
4. the ferric chloride seals the blood vessel
Brownian movement is caused by:
1. Colloidal solution.
2. True solution.
3. Suspension having size <1000 m.
4. All of the above.
A dust storm is:
1. Dispersion of solid in gas
2. Dispersion of a gas in solid
3. Dispersion of solid in solid
4. Dispersion of a gas in liquid
Which is not a colloid?
1. Chlorophyll
2. Egg white
3. Ruby glass
4. Milk






