The correct acidity order of the following is:


1. (III)>(IV)>(II)>(I)
2. (IV)>(III)>(I)>(II)
3. (III)>(II)>(I)>(IV)
4. (II)>(III)>(IV)>(I)


Acetone reacts with iodine (I2) to form iodoform in the presence of
1. CaCO3
2. NaOH
3. KOH
4. MgCO3
Generally Aldehydes behave as:
1. Oxidising agent
2. Reducing agent
3. Dehydration agent
4. Oxidizing as well as reducing agent
A and B in the following reactions are


1. A=RR'CH2CN, B=NaOH
2. A=RR'C , B=CH3
3. A=RR'C , B=CH3
4. A=RR"C, B=LiAlH4
RCOOH RCH2OH. This mode of reduction of an acid to alcohol can be affected only by:
1. Zn/HCl
2. Na-alcohol
3. aluminium isopropoxide and isopropyl alcohol
4. LiAlH4
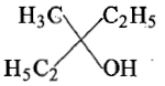
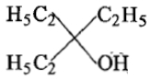
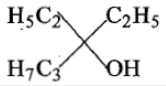
Ethyl ester P. the product P will be:
1. 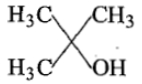

2. 

3. 

4. 

Which one of the following can be oxidised to the corresponding carbonyl compound? [2004]
1. 2-hydroxy propane
2. Ortho-nitro phenol
3. propane
4. 2-methyl-2-hydroxy propane
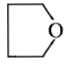
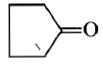
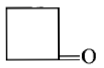
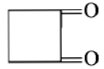
Dry distillation of barium salt of Hexane-1,6-dicarboxylic acid gives:
1. 

2. 

3. 

4. 

Polarisation of electrons in acrolein may be written as
1.
2.
3.
4.
Consider the following reaction;
CH3Br + Mg ABC
compound C is:
1. acetic acid
2. acetaldehyde
3. ethyl alcohol
4. formic acid






