A and B in the following reactions are


1. A=RR'CH2CN, B=NaOH
2. A=RR'C , B=CH3
3. A=RR'C , B=CH3
4. A=RR"C, B=LiAlH4


Acetaldehyde normally reacts with
1. only electrophiles
2. only nucleophiles
3. only free radicals
4. both electrophiles and nucleophiles
Nucleophilic addition reaction will be most favoured in [2006]
1.
2.
3.
4.
The enolic form of acetone contains:
1. 9 -bonds, 1-bond and 2 lone pairs
2. 8 -bonds, 2-bonds and 2 lone pairs
3. 10 -bonds, 1-bond and 1 lone pair
4. 9 -bonds, 2-bonds and 1 lone pair
In this reaction,
CH3CHO + HCN CH3CH(OH)CN CH3CH(OH)COOH
an asymmetric compound is generated. The acid obtained would be
1. 50% D + 50% L-isomer
2. 20% D + 80% L-isomer
3. D-isomer
4. L-isomer
Formaldehyde can be distinguished from acetaldehyde by:
1. Fehling's solution
2. Schiff's reagent
3. Ammonia
4. Ammoniacal


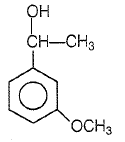
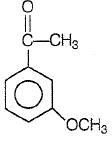
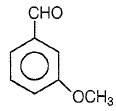
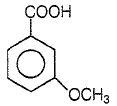
Product P in the above reaction is -
1. 

2. 

3. 

4. 

Acetone reacts with iodine (I2) to form iodoform in the presence of
1. CaCO3
2. NaOH
3. KOH
4. MgCO3
The -OH group of an alcohol or the -COOH group of a carboxylic acid can be replaced by -Cl using
1. phosphorus pentachloride
2. hypochlorous acid
3. chlorine
4. hydrochloric acid
Which one of the following can be oxidised to the corresponding carbonyl compound?
1. 2-hydroxy propane
2. Ortho-nitro phenol
3. Phenol
4. 2-methyl-2-hydroxy propane






