RCOOH →→RCH2OH. This mode of reduction of an acid to alcohol can be affected only by:
1. Zn/HCl
2. Na-alcohol
3. aluminium isopropoxide and isopropyl alcohol
4. LiAlH4
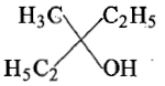
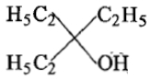
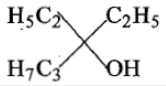
Ethyl ester CH3MgBr→ExcessCH3MgBr−−−−−→Excess P. the product P will be:
1. 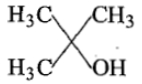

2. 

3. 

4. 

Which of the following has most acidic proton?
1. CH3COCH3
2. (CH3)2C=CH2
3. CH3COCH2COCH3
4. (CH3.CO)3CH
Which one of the following can be oxidised to the corresponding carbonyl compound? [2004]
1. 2-hydroxy propane
2. Ortho-nitro phenol
3. propane
4. 2-methyl-2-hydroxy propane
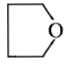
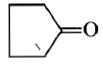
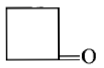
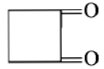
Dry distillation of barium salt of Hexane-1,6-dicarboxylic acid gives:
1. 

2. 

3. 

4. 

Polarisation of electrons in acrolein may be written as
1. δ+CH2=CH-CH=δ-Oδ+CH2=CH−CH=δ−O
2. δ+CH2=δ+CH-CH=Oδ+CH2=δ+CH−CH=O
3. δ+CH2=CH-CH=δ+Oδ+CH2=CH−CH=δ+O
4. δ+CH2=CH-δ+CH=Oδ+CH2=CH−δ+CH=O
Consider the following reaction;
CH3Br + Mg Ether→Ether−−→AHCHO→HCHO−−−→BHOH→HOH−−→C
compound C is:
1. acetic acid
2. acetaldehyde
3. ethyl alcohol
4. formic acid
The conversion of CH3OH to CH3COOH can be brought in by:
1. K2Cr2O7/H+
2. CO + Rh
3. KMnO4
4. H3PO4
Benzaldehyde and acetaldehyde can be distinguished by:
1. Iodoform test
2. 2,4 - DNP test
3. NH3NH3 reaction
4. Wolff-Kishner's reduction
Fehling's solution is:
1. Acidified copper sulphate solution
2. Ammoniacal cuprous chloride solution
3. Copper sulphate, Rochelle salt + NaOH
4. None of the above






