Aniline is reacted with bromine water and the resulting product is treated with an aqueous solution of sodium nitrite in presence of dilute hydrochloric acid. The compound so formed is converted into a tertafluoroborate which is subsequently heated. The final product is
1. 1,3,5-tribromobenzene
2. p-bromofluorobenzene
3. p-bromoaniline
4. 2,4,6-tribromofluorobenzene
Alkyl halide (RX) on treatment with KCN followed by reduction leads to the formation of:
1. RNH2
2. RCH2NH2
3. RH + NH3
4. RCH3 + N2
The end product in the following sequence of reactions is -
1. Ethyl cyanide
2. Ethylamine
3. Methylamine
4. Acetamide
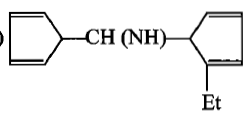
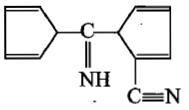
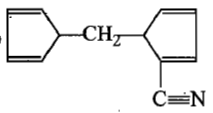
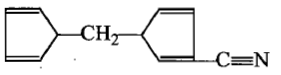
The product[A] formed in the reaction;
1. 

2. 

3. 

4. 

Identfy X in the sequence,
:
1.
2.
3.
4. none of the above
CH3CH2NH2 contains a basic NH2 group, but CH3CONH2 does not, because;
1. acetamide is amphoteric in character
2. in CH3CH2NH2 the electron pair on N-atom is delocalized by resonance
3. in CH3CH2NH2 there is no resonance, while in acetamide the lone pair of electron on N-atom is delocalized and therefore less available for protonation
4. none of the above
Dehydration of an amide gives:
1. Cyanide
2. Amine
3. Isocyanide
4. Fatty acid
Tertiary nitro compounds cannot show tautomerism because:
1. they are very stable
2. isomerises to give sec. nitro compounds
3. do not have labile H-atom
4. they are highly eactive
A secondary amine is:
1. a compound with two -NH2 groups
2. a compound with 2 carbon atoms and a -NH2 group
3. a compound with a -NH2 group on the carbon atom in number 2 position
4. a compound in which 2 of the hydrogens of NH3 have been replaced by alkyl or aryl groups
Compound that will undergo Hoffmann reaction is-
1. RCONHCH3
2. RCOONH4
3. RCONH2
4. RCONHOH






