The bond energy of H—H and Cl-Cl is 430 kJ
mol-1 and 240 kJ mol-1 respectively and ΔHf for HCl is -90 kJ mol-1. The bond enthalpy of HCl is:
1. 290
2. 380
3. 425
4. 245
mol-1 and 240 kJ mol-1 respectively and ΔHf for HCl is -90 kJ mol-1. The bond enthalpy of HCl is:
A gas behaving ideally was allowed to expand reversibly and adiabatically from 1 litre to 32 litre. It's initial temperature was 327C. The molar enthalpy change (in J/mol) for the process is:
1. -1125 R
2. -675
3. -1575 R
4. None of these
2 mole of an ideal monoatomic gas undergoes a reversible process for which PV2=C. The gas is expanded from initial volume of 1 L to final volume of 3 L starting from initial temperature of 300 K. Find for the process:
1. -600 R
2. -1000 R
3. -3000 R
4. None of these
Calculate for 3 mole of a diatomic ideal gas which is heated and compressed from 298 K and 1 bar to 596 K and 4 bar: [Given: Cv, m (gas)= R; ln (2)=0.70; R=2 cal k-1 mol-1]
1. -14.7 cal K-1
2. +14.7 cal K-1
3. -6.9 cal K-1
4. 6.3 cal K-1
For the hypothetical reaction
are 20 kJ/mol and -20 JK-1 mol-1 respectively at 200 K.
If is 20 JK-1 mol-1 then at 400 K is:
1. 20 kJ/mol
2. 7.98 kJ/mol
3. 28 kJ/mol
4. None of these
Calculate (in kJ/mol) for from the and the values provided at 27C
\(\begin{array}{ll}
4 C r(s)+3 O_2(g) \rightarrow 2 C r_2 O_3(s), \\ \Delta_r G^{\circ}=-2093.4 k J / m o l \\
S^{\circ}(\mathrm{J} / / \mathrm{K} \mathrm{~mol}): S^{\circ}(C r, s)=24, \\ S^{\circ}\left(O_2, g\right)=205, \quad S^{\circ}\left(C r_2 O_3, s\right)=81
\end{array}\)
1. -2258.1 kJ/mol
2. -1129.05 kJ/mol
3. -964.35 kJ/mol
4. None of the above
Boron can undergo the following reactions with the given enthalpy changes: 2B(s)
Assume no other reactions are occurring. If in a container (operating at constant pressure) which is isolated from the surrounding, a mixture of H2(gas) and (gas) is passed over excess of B(s), then calculate the molar ratio () so that the temperature of the container does not change :
1. 15:3
2. 42:1
3. 1:42
4. 1:84
Consider the reaction at 300 K . What is AU for the combustion of 1.5 moles of benzene at 27°C
1. -3267.25 kJ
2. -4900.88 kJ
3. -4906.5 kJ
4. -3274.75 kJ
A gas expands against a variable pressure given by P = 20/V (where P in atm and V in L). During expansion from the volume of 1 liter to 10 liters, the gas undergoes a change in internal energy of 400 J. How much heat is absorbed by the gas during expansion?
1. 46 J
2. 4660 J
3. 5065.8 J
4. 4260 J
An ideal gas is taken around the cycle ABCA as shown in P-V diagram. The network done during the cycle is equal to :
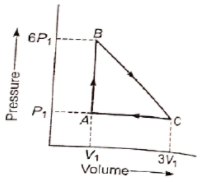
1. 12
2. 6
3. 5
4.






