The incorrect method for the preparation of Me3COMe in good yield is
1. Me3CCl + MeONa →
2. \(Me_{2}C=CH_{2}\xrightarrow[(ii)NaBH_{4}]{(i) Hg(OAc)_{2}/MeOH}\)
3. Me3CONa + MeCl ⟶
4. \(Me_{2}C=CH_{2}\xrightarrow[MeOH]{H^{+}}\)
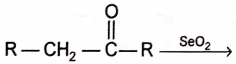 Product of products
Product of products
1. R - COOH only
2. R - CHO only
3. R - CHO and R - COOH
4.
1. Three
2. Four
3. Two
4. One
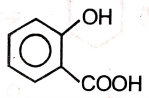
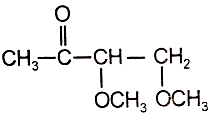
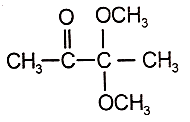
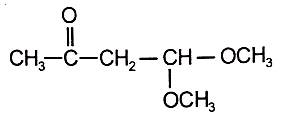
An organic compound (A) of the molecular formula gives a positive Iodoform test but does not reduce Tollen's reagent. When (A) is subjected to acid-catalyzed hydrolysis, another compound B is formed which gives positive Tollen's test. Identify the unknown compound (A).
1. 
2. 
3. 
4. 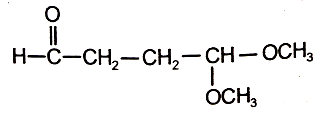
Product (A) can give
1. Haloform test
2. Aldol condensation
3. Cannizaro reaction
4. Both (A) & (B)
Consider the following reaction,
The B and C are respectively:
1. RCHO and HCHO
2. RCOOH and HCOOH
3. RCH2OH and CH3OH
4.
The values of some of the acids are given below:
| Acid | \(\mathrm{pK_a}\) | Acid | \(\mathrm{pK_a}\) |
 |
-0.6 | HI | -10.0 |
 |
4.8 | \(\mathrm{HC} \equiv \mathrm{CH}\) | 25 |
| \(\overset{+}{N}H_4\) | 9.4 | \(H_2S\) | 7.0 |
The correct order of leaving tendency of their conjugate bases is:
| 1. | 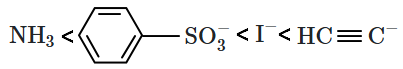 |
| 2. |  |
| 3. |  |
| 4. |  |
Among the following esters. form a pair of least reactive and most reactive ester towards hydrolysis in that order
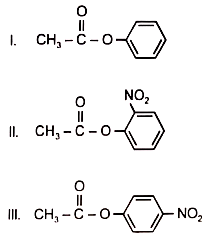
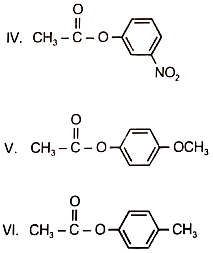
1. V. III
2. VI, II
3. I, IV
4. I, V




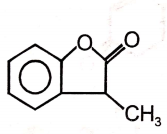 2.
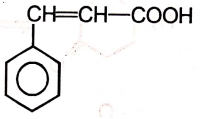
2. 
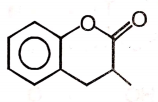 4.
4.