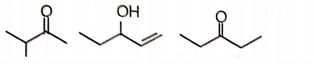
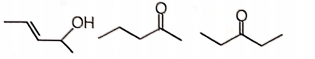
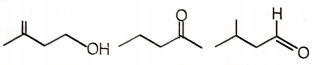
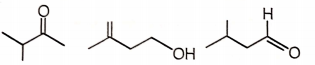
Three isomeric compounds M, N and P () give the following tests
i. M and P react with sodium bisulfite to form an adduct.
ii. N consumes 1 mol of bromine and also gives turbidity with conc. HCl/anhydrous after prolonged heating.
iii. M reacts with an excess of iodine in an alkaline solution to give a yellow crystalline compound with a characteristic smell.
iv. p-Rosaniline treated with sulfur dioxide develops pink color on shaking with P.
The structures of M, N, and P, respectively are
M N P
1. 
2. 
3. 
4. 





To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The major product in the following reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The main product X formed in the following reaction is
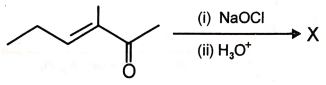
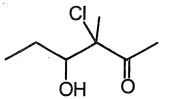
1. 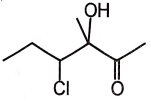 2.
2. ![]()
3. ![]() 4.
4. 

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The major product formed in the following reaction is:

| 1. |  |
2. |  |
| 3. |  |
4. | 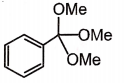 |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
In the following reactions
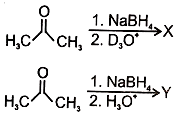
1. 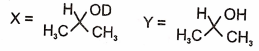
2. 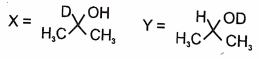
3. 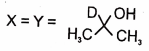
4. 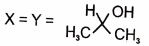

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
In the given below reaction
x, y, and z are:
1. x = Mg; dry ether; y =
2. x = Mg; dry methanol;
3. x = Mg; dry ether;
4. x = Mg; dry methanol;

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
4-Formylbenzoic acid on treatment with one equivalent of hydrazine followed by heating with alcoholic KOH gives, as major product:
| 1. |  |
2. |  |
| 3. | 4. |  |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
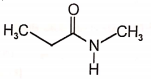
The major final product in the following reaction is
1. ![]() 2.
2. ![]()
3. 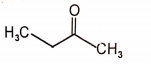 4.
4. 
The reddish-brown precipitate formed in Fehling's test for aldehydes (RCHO) is due to the formation of the following compound :
1. \(\mathrm{Cu}\)
2. \(\mathrm{Cu}_2 \mathrm{O}\)
3. \(\mathrm{CuO}\)
4. \((\mathrm{RCOO})_2 \mathrm{Cu}\)
The correct order of acidity of the following compounds is:
| 1. | 1 > 2 > 3 | 2. | 1 > 3 > 2 |
| 3. | 3 > 1 > 2 | 4. | 3 > 2 > 1 |









