The major product in the following reaction is:

1.

2.

3.

4.







To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
‘D’ would be





To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Xylene on oxidation with acidic gives:
1. Phthalic acid
2. Isophthalic acid
3. Terephthalic acid
4. All of the above

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The major product obtained when is reacted with

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Identify X in the following sequence of reactions :
\(\mathrm{X} \xrightarrow[\text { (ii) } \mathrm{H}^{+} / \mathrm{H}_2 \mathrm{O}]{\text { (i) } \mathrm{CH}_3 \mathrm{MgX}} \mathrm{C}_5 \mathrm{H}_{12} \mathrm{O} \xrightarrow[573 \mathrm{~K}]{\mathrm{Cu}} \mathrm{C}_5 \mathrm{H}_{10}\)
| 1. | \(\mathrm{CH}_3 \mathrm{COCH}_2 \mathrm{CH}_3\) |
| 2. | \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CHO}\) |
| 3. | \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CHO}\) |
| 4. | \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{OH}\) |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
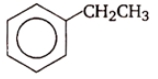
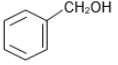
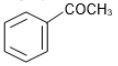
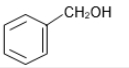
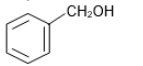
The reaction that does not yield benzoic acid as the major product is:
1. 
2. 
3. 
4. 

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the following will not form a yellow precipitate on heating with an alkaline solution of iodine?
1. CH3CH(OH)CH3
2. CH3CH2CH(OH)CH3
3. CH3OH
4. CH3CH2OH

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
On warming with I2 and aqueous NaOH, iodoform and sodium succinate are formed. The formula of the compound should be-
1. CH3COCH2CH2CH3
2. CH3COC6H5
3. CH3COCH2CH2COCH3
4. None of the above

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

Product is
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.





 is
is






