Which of the following compounds will exhibit cis-trans (geometrical) isomerism?
1. 2-butene
2. Butanol
3. 2-butyne
4. 2-butenol

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The IUPAC name of the compound having the formula CH≡C–CH=CH2 is :
1. 3-Butene-1-yne
2. 1-Butyn-3-ene
3. But-1-yne-3-ene
4. Buten-3-yne

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
| 1. |  |
2. |  |
| 3. |  |
4. |  |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Decreasing order of acidic character for cyclohexanol (I), acetic acid (II), 2, 4, 6-trinitrophenol (III) and phenol (IV), will be
1. III>II>IV>I
2. II>III>I>IV
3. II>III>IV>I
4. III>IV>II>I

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which one of the following is most reactive towards electrophilic reagent?
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The correct IUPAC name of the following compound is:

1. 3-Ethyl-4-ethenylheptane
2. 3-Ethyl-4-propylhex-5-ene
3. 3-(1-Ethyl propyl)hex-1-ene
4. 4-Ethyl-3-propylhex-1-ene

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The correct order of increasing bond length of
C-H, C-O, C-C and C=C is
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Considering the state of hybridization of carbon atoms, find out the molecules among the following which is linear.
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.




To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
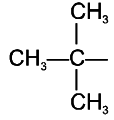

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.










