(CH3)4N+ is neither an electrophile nor a nucleophile because of it:
1.
Does not have an electron pair for donation and it also cannot attract an electron pair.
2.
Neither has an electron pair available for donation nor can accommodate electrons since all shells of N are fully occupied.
3.
Can act as Lewis acid and base.
4.
None of the above.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the following explains the greater stability of 2,3-dimethylbut-2-ene compared to 2-butene?
1. Resonance
2. Hyperconjugation
3. Electromeric effect
4. Inductive effect

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Among the following the most stable carbocation is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Silver sulphate solution is used to separate:
1. Nitrate and bromide
2. Nitrate and chlorate
3. Bromide and iodide
4. Nitrate and nitrite

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The IUPAC name of 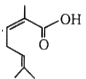 is:
is:
1. 2,6-Dimethylhepta-2, 5-dienoic acid
2. 3,7-Dimethylhepta-2, 5-dienoic acid
3. 1-Hydroxy-2, 6-dimethylhepta-2, 5-dienone
4. None of the above

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The IUPAC name of the given compound is:
1. Ethyl 2-methylprop-2-enoate
2. Ethyl 2-methylprop-1-enoate
3. 1-Ethoxy 2-methylprop-2-enoate
4. 1-Ethoxy 2-methylprop-2-enal

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
A compound that does not give a positive test in Lassaigne’s test for nitrogen is-
| 1. | Urea | 2. | Hydrazine |
| 3. | Azobenzene | 4. | Phenyl hydrazine |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
In Kjeldahl's method of estimation of nitrogen, K2SO4 acts as:
1. An oxidising agent
2. Catalytic agent
3. Hydrolysing agent
4. Boiling point elevator
What colour is produced when sodium nitroprusside is added to an alkaline solution of sulfide ions?
1. Red
2. Blue
3. Brown
4. Purple

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which structure demonstrates the highest stability?
| 1. |  |
2. |  |
| 3. |  |
4. | 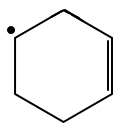 |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.







