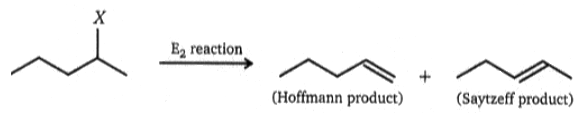
(A)In the above reaction, maximum Saytzeff product will obtained when
(a) X = I (b) X = -Cl (c) X = -Br (d) X = -F
(B) In the above reaction Hofmann product is major when X is:
(a) -I (b) -Cl (c) -Br (d) -F
1. A- a , B - d
2. A- b, B - c
3. A -c , B - a
4. A - d, B -d

(a) X = I (b) X = -Cl (c) X = -Br (d) X = -F
(a) -I (b) -Cl (c) -Br (d) -F
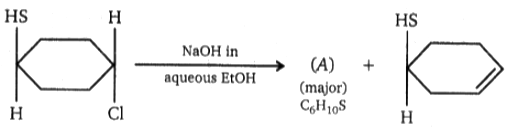
Major product of the above reaction is:
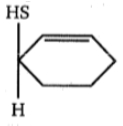
1. 
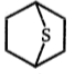
2. 
3. ![]()
4. 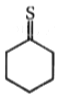
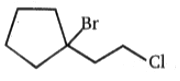
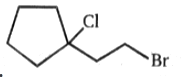
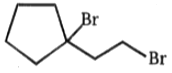
Major product is :-
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Addition of KI accelerates the hydrolysis of primary alkyl halides because:
1. KI is soluble in organic solvents
2. The iodide ion is a weak base and a poor leaving group
3. The iodide ion is a strong base
4. The iodide ion is a powerful nucleophile as well as a good leaving group
Consider the nucleophilic attacks given below. Select in each pair that shows the greater SN2 reaction rate.
(A) 
(B) 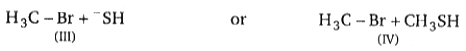
(C) 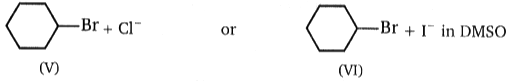
(D) 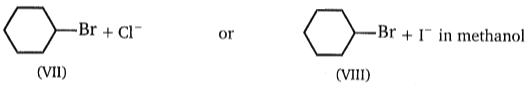
A B C D
1. II IV VI VIII
2. II III V VIII
3. I III V VIII
4. I III V VII

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
What is the major product of the following reaction?
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
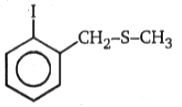
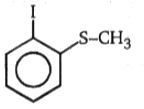
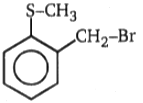
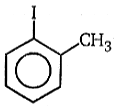 (A)(B), Product (B) is:
(A)(B), Product (B) is:
1. 
2. 
3. 
4. None of the above
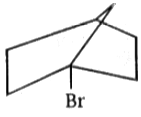
Which of the following reactant will not favour nucleophilic substitution reaction ?
1. 
2. Ph-Br
3.
4. All of the above
Rank the following in order of decreasing rate of solvolysis with aqueous ethanol (fastest slowest)
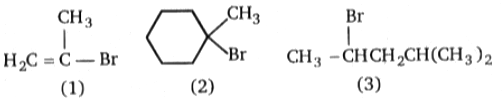
1. 2>1>3
2. 1>2>3
3. 2>3>1
4. 1>3>2

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
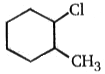
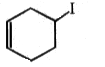
Which of the following alkyl halide undergo rearrangement in SN1 reaction?
1.
2. 
3. 
4. All of these

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.








