To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
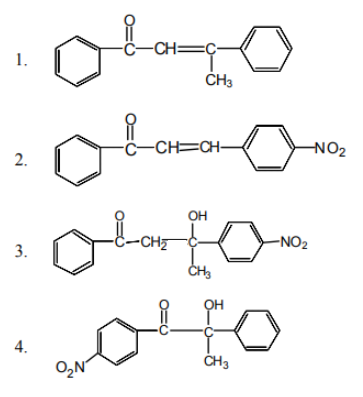
The correct order of increasing reactivity towards
acid hydrolysis is
1. 1 < 2 < 3
2. 3 < 1 < 2
3. 1 < 3 < 2
4. 2 < 1 < 3
I. R1 = H II R1 = CH3
R2 = CH3 R2 = -CH(CH3)2
III. R1 = CH3 IV R1 = CH3
R2 = CH3 R2 = C2H5
The rate of addition follows the order
1. I > II > III > IV
2. I > III > IV > II
3. I > III > II > IV
4. I > II > IV > III

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The end product of the following reaction would be
Which acid is least reactive to decarboxylation when heated?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
When glycerol is reacted with oxalic acid at 260oC, then the final product formed is
1. Formic acid
2. Allyl alcohol
3. Acrolein
4. Acetic Acid
When propanoic acid is treated with aqueous sodium bicarbonate, CO2 is liberated. The ‘C’ of CO2 comes from:
1. Carboxylic acid group
2. Methylene group
3. Bicarbonate
4. Methyl group

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
In the reaction
the oxidizing agent can be
1. Alkaline KMnO4
2. Acidified K2Cr2O7
3. Benedict’s solution
4. All of the above
The appropriate reagent for the transformation is
1. Zn (Hg)/HCl
2. NH2–NH2/OH–
3. Both 1. and 2.
4. None of the above

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
In a Cannizzaro’s reaction, the intermediate that will
be the best hydride donor is