In the reaction
the oxidizing agent can be
1. Alkaline KMnO4
2. Acidified K2Cr2O7
3. Benedict’s solution
4. All of the above
The appropriate reagent for the transformation is
1. Zn (Hg)/HCl
2. NH2–NH2/OH–
3. Both 1. and 2.
4. None of the above

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
In a Cannizzaro’s reaction, the intermediate that will
be the best hydride donor is
What is the product (Y) formed in the following sequence of reactions?

| 1. |  |
2. |  |
| 3. |  |
4. |  |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
An organic compound (A) (MF C4H10O) upon dehydrogenation gives a compound (B) which forms phenyl hydrazone with phenylhydrazine and both the compounds (A) and (B) respond to iodoform test. Hence, the compound (A) is

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The major product of the following reaction is:
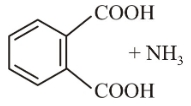
1.
2.
3.
4.
Self condensation of two moles of ethyl acetate in presence of sodium ethoxide yields :
1. Ethyl butyrate
2. Acetoacetic ester
3. Methyl acetoacetate
4. Ethyl propionate
Nucleophilic addition reaction will be most favoured in-
1.
2.
3.
4.
A carbonyl compound reacts with hydrogen cyanide to form cyanohydrins which on hydrolysis forms a racemic mixture of α-hydroxy acid. The carbonyl compound is :
1. Acetaldehyde
2. Acetone
3. Diethyl ketone
4. Formaldehyde
In a set of reactions propanoic acid yielded a compound D.
The structure of D would be :
1.
2.
3.
4.













