Among the given compounds, the most susceptible to nucleophilic attack at the carbonyl group is
1.
2.
3.
4.
Which one is a nucleophilic substitution reaction among the following?
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
In a set of reactions, m-bromobenzoic acid gave a product D. The product D is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Clemmensen reduction of a ketone is carried out in the presence of which of the following?
1. - with
2.
3. and as catalyst
4. Glycol with

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
and can be distinguished chemically by-
1. Benedict test
2. Iodoform test
3. Tollen's reagent test
4. Fehling solution test

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The correct order of decreasing acid strength of trichloroacetic acid (A), trifluoroacetic acid (B), acetic acid (C) and formic acid (D)
1. B > A > D > C
2. B > D > C > A
3. A > B > C > D
4. A > C > B >D

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.






To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
| 1. | |
| 2. | |
| 3. | |
| 4. |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
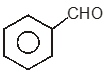
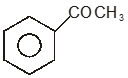
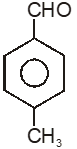
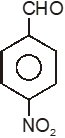

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
2. Cyclopentanonyl cation
3. Cyclopentanonyl radical
4. Cyclopentanonyl biradical

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.







