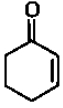
Reaction by which Benzaldehyde cannot be prepared:
1.
 + H2 in presence of Pd-BaSO4
+ H2 in presence of Pd-BaSO4
2.
 + CO + HCl in presence of anhydrous AlCl3
+ CO + HCl in presence of anhydrous AlCl3
3.
 + Zn/Hg and conc. HCl
+ Zn/Hg and conc. HCl
4.
 + CrO2Cl2 in CS2 followed by H3O+
+ CrO2Cl2 in CS2 followed by H3O+
| 1. | |
| 2. | |
| 3. | |
| 4. |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
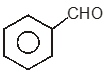
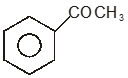
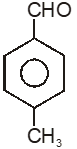
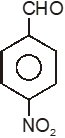

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
2. Cyclopentanonyl cation
3. Cyclopentanonyl radical
4. Cyclopentanonyl biradical

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Reaction of a carbonyl compound with one of the following reagents involves nucleophilic addition followed by the elimination of water. The reagents is
1. A Grignard reagent
2. Hydrazine in presence of feebly acidic solution
3. Hydrocyanic acid
4. Sodium hydrogen sulphite

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.




2. Carboxylic acid
3. Aromatic acid
4. Schiff base

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The correct order of strengths of the carboxylic acids
1. I>II>III
2. II>III>I
3. III>II>I
4. II>I>III

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Compound A, is found to react with NaOI (produced by reacting Y with NaOH) and yields a yellow precipitate with characteristic smell.
A and Y are respectively
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Carboxylic acids have higher boiling points than aldehydes, ketones and even alcohols of comparable molecular mass. It is due to their
1. Formation of intramolecular H-bonding
2. Formation of carboxylate ion
3. More extensive association of carboxylic acid via van der waals force of attraction
4. Formation of intermolecular H-bonding

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.