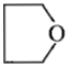
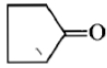
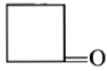
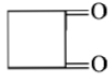
Dry distillation of barium salt of Hexane-1,2-dicarboxylic acid gives:
1. 
2. 
3. 
4. 




Ethyl ester P.
The product P will be
| 1. |  |
2. |  |
| 3. |  |
4. |  |
RCOOH RCH2OH. This mode of reduction of an acid to alcohol can be affected only by:
1. Zn/HCl
2. Na-alcohol
3. aluminium isopropoxide and isopropyl alcohol
4. LiAlH4

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Compound (A) C5H10O forms a phenyl hydrazone and gives negative Tollen's and iodoform tests. Also, compound (A) on reduction gives n-pentane. Compound (A) is-
| 1. | A primary alcohol | 2. | An aldehyde |
| 3. | A ketone | 4. | A secondary alcohol |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Formaldehyde can be distinguished from acetaldehyde by:
1. Fehling's solution
2. Schiff's reagent
3. Ammonia
4. Ammoniacal
What is the chemical reaction of lachrymator or tear gas?
1. C6H5COCl
2. C6H5OC6H5
3. C6H5COCH2Cl
4. C6H5COCH3
HCHO and HCOOH are distinguished by treating with:
1. Tollens reagent
2. NaHCO3
3. Fehling's Solution
4. Benedict Solution

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
A compound that gives two different alcohols on reduction with is:
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The compound formed when malonic acid heated with urea, is-
1. Cinnamic acid
2. Butyric acid
3. Barbituric acid
4. Crotonic acid

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
An ester is boiled with KOH. The product is cooled and acidified with conc. HCl. A white crystalline acid separates. The ester is-
1. Methyl acetate
2. Ethyl acetate
3. Ethyl formate
4. Ethyl benzoate

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.






