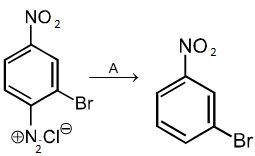
 In the above reaction, A is:
1. Cu2Cl2
In the above reaction, A is:
1. Cu2Cl2
2. H3PO2 and H2O
3. H+ / H2O
4. HgSO4 / H2SO4

2. H3PO2 and H2O
3. H+ / H2O
4. HgSO4 / H2SO4

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Among the following options, which diazonium salt would be the most stable?
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
 +
+ 


To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
2. Azoxybenzene
3. Azobenzene
4. Aniline
Method by which aniline cannot be prepared is
1. Hydrolysis phenyl isocyanide with acidic solution
2. Degradation of benzamide with bromine in alkaline Solution
3. Reduction of nitrobenzene with H/Pd in ethanol
4. Potassium salt of phthalimide treated with chlorobenzene followed by the hydrolysis aqueous NaOH solution

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
| 1. | Arylamines are generally more basic than alkylamines because the nitrogen lone-pair electrons are not delocalized by interaction with the aromatic ring -electron system. |
| 2. | Arylamines are generally more basic than alkylamines because of aryl group. |
| 3. | Arylamines are generally more basic than alkylamines because the nitrogen atom in arylamines is sp-hybridized. |
| 4. | Arylamines are generally less basic than alkylamines because the nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring -electron system. |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
A given nitrogen-containing aromatic compound A reacts with Sn/HCl, followed by HNO2 to give an unstable compound B. B, on treatment with phenol, forms a beautiful coloured compound C with the molecular formula C12H10N2O . The structure of compound A is-
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which one of the following nitro-compounds does not react with nitrous acid
1.
2.
3.
4.
Nitration of aniline in strong acidic medium also gives m-nitroaniline because
1. In spite of substituents nitro group always goes to only m-position
2. In electrophilic substitution reactions amino group is meta directive
3. In absence of substituents nitro group always goes to only m-position
4. In acidic (strong) medium aniline is present as anilinium ion

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.


To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.














