The compounds containing sp hybridized carbon atoms are:
i.

ii.

iii.
H3C-CN
iv.
H2C=C=CHCH3
1. i and ii
2. iii and iv
3. ii and iii
4. i and iv


1. i and ii

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
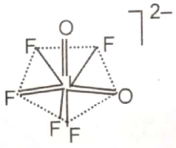
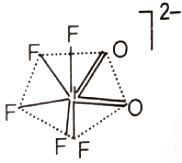
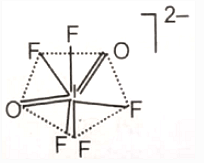
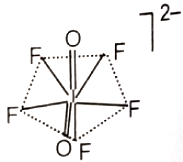
The hybridization of the central atom and the shape of [lO2F5]2- ion, respectively, are
1. sp3d3
2. sp2d4
3. sp2d4
4. sp3d3
Which of the following contain a maximum number of electrons in the antibonding molecular orbitals?
| 1. | O2−2 | 2. | O2 |
| 3. | O−2 | 4. | O+2 |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the following compounds has the least tendency to form hydrogen bonds between molecules?
1. NH3
2. H2NOH
3. HF
4. CH3F

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Assuming that Hund's rule is violated by the diatomic molecule B2, its bond order and magnetic nature will be respectively
1. 1, diamagnetic
2. 1, paramagnetic
3. 2, diamagnetic
4. 2, paramagnetic

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
In the Lewis structure of ozone (O3) the formal charge on the central oxygen atom is:
1. +1
2. -1
3. 0
4. -2

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The types of hybrid orbitals of nitrogen in NO+2, NO-3, and NH+4 respectively, are expected to be:
1. sp, sp3 and sp2
2. sp, sp2 and sp3
3. sp2, sp and sp3
4. sp2 , sp3 and sp

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the following species has tetrahedral geometry(shape)?
1. BH-4
2. NH-2
3. CO2-3
4. H3O+

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which molecule /ion out of the following does not contain unpaired electrons?
1. N+2
2. O2
3. O2-2
4. B2

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the following order of energies of molecular orbitals of N2 is correct?
1. (π2py) < (σ2pz) < (π*2px) ≈ (π*2py)
2. (π2py) > (σ2pz) > (π*2px) ≈ (π*2py)
3. (π2Py) < (σ2pz) > (π*2px) ≈(π2py)
4. (π2py) > (σ2pz) < (π*2px) ≈(π*2py)

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.







