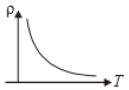
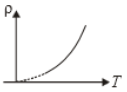
The variation of density (p) of gas with its absolute temperature (T) at constant pressure is best represented by the graph
1. 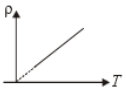 2.
2. 
3. 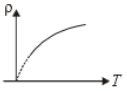 4.
4. 
 2.
2. 
 4.
4. 
An ideal gas changes from state 'a' to state 'b' as shown in figure. What is the work done by the gas in the process?
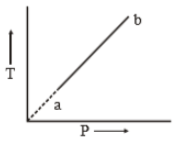
1. zero
2. positive
3. negative
4. infinite
The temperature inside a refrigerator is and the room temperature is . The amount of heat delivered to the room for each joule of electrical energy consumed ideally will be -
(1)
(2)
(3)
(4)
A refrigerator works between 4°C and 30°C. It is required to remove 600 calories of heat every second in order to keep the temperature of the refrigerated space constant. The power required is (Take, 1 cal = 4.2 Joules)
(1) 23.65W
(2) 236.5W
(3) 2365W
(4) 2.365W
The figure below shows two paths that may be taken by a gas to go from state A to state C. In process AB, \(400~\text{J}\) of heat is added to the system and in process BC, \(100~\text{J}\) of heat is added to the system. The heat absorbed by the system in the process AC will be:
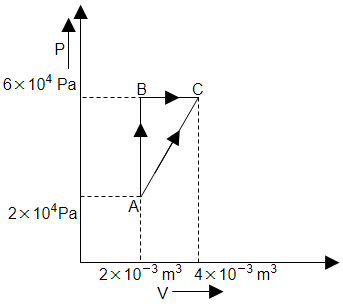
| 1. | \(380~\text{J}\) | 2. | \(500~\text{J}\) |
| 3. | \(460~\text{J}\) | 4. | \(300~\text{J}\) |
The coefficient of performance of a refrigerator is 5. If the temperature inside freezer is -20°C, the temperature of the surroundings to which it rejects heat is -
(1)31°C
(2)41°C
(3)11°C
(4)21°C
One mole of an ideal gas from an initial state A undergoes via two processes. It first undergoes isothermal expansion from volume V to 3V and then its volume is reduced from 3V to V at constant pressure. The correct p-V diagram representing the two processes is -
An ideal gas goes from state A to state B
via three different processes as indicated
in the p-V diagram
If indicates the heat absorbed
by the gas along the three processes and
indicates the change in
internal energy along the three processes
respectively, then
(a)
(b)
(c)
(d)
A container of volume 1m3 is divided into two equal compartments by a partition. One of these compartments contains an ideal gas at 300 K. The other compartment is vaccum. The whole system is thermally isolated from its surroundings. The partition is removed and the gas expands to occupy the whole volume of the container. Its temperature now would be -
(1) 300 K
(2) 239 K
(3) 200 K
(4) 100 K
A thermo-dynamical system is changed from state (P1, V1) to (P2, V2) by two different process. The quantity which will remain same will be
(1) ΔQ
(2) ΔW
(3) ΔQ + ΔW
(4) ΔQ – ΔW








