The bond order depends on the number of electrons in the bonding and antibonding orbitals. Which of the following statements are correct about bond order?
1. Bond order can have a negative value.
2. It always has an integral value.
3. It is a nonzero quantity
4. It can assume any value-positive or integral or fractional, including zero upto four.
What is the best description of the geometry of the nitrogen atoms in dimethylnitrosamine, ?
N bounded to groups N bonded to O
1. Trigonal planar Linear
2. Trigonal planar Bent
3. Trigonal pyramidal Linear
4. Trigonal pyramidal Bent
The following diagram is sometimes used to illustrates the structure of benzene
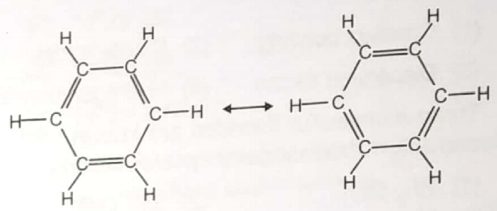
Which of the statements concerning the structure of benzene is false?
1. The double bonds oscillate rapidly back and forth between adjacent pairs of carbon atoms
2. The
3. The carbon atoms form a flat hexagonal ring
4. Above structures are resonance structures
Consider the Lewis structure given below for the molecule
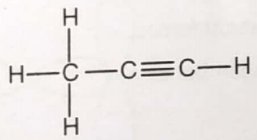
What is the maximum number of atoms that can lie in the same plane?
1. Three
2. Four
3. Five
4. Six
How many carbon-carbon double bonds are present in linolenic acid, ?
1. 1
2. 2
3. 3
4. 4
The formulae and boiling points of three compounds are given in this table.
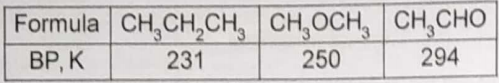
The trend in boiling points is best attributed to variations in
1. Covalent bonding
2. Dipole forces
3. Dispersion forces
4. Hydrogen bonding
Three monosulfur fluorides are known : . Of these, polar species include
1. Only
2. Only
3. and Only
4. , and
Which ionic compound has the largest lattice energy?
1. LiF
2. BeO
3. KBr
4. CaS
The boiling points of are , respectively. This increase is best attributed to an increase in which of the following?
l. Dipole-dipole interactions.
ll. Dispersion forces.
lll. Hydrogen bonding.
1. l only
2. l and ll.
3. lll only
4. ll and lll only






