How can this reaction is made to proceed in forward direction?
1. Addition of cis-1, 2 diol
2. Addition of borax
3. Addition of trans-1, 2 diol
4. Addition of
Which of the following compound(s) can react with ?
1. Ethers
2.
3.
4. All of the above
Which of the following statement is incorrect regarding B-F bond in ?
1. All the three B-F bond lengths are equal and each of them is shorter than the sum of the covalent radii of boron and fluorine
2. The bond energy of the B-F bond is very high, higher than for any other single bond
3. The unusual shortness and strength of the B-F bond may be explained by interaction between boron and fluorine atoms
4. The unusual shortness and strength of the bonds may be explained by a interaction between boron and fluorine atoms
Under hydrolytic conditions, the compounds used for the preparation of the linear polymer and for chain termination, respectively, are
1.
2.
3.
4.
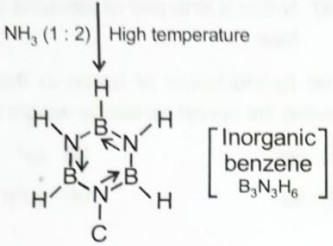
Select the incorrect statement regarding compounds (X), (Y) and (Z).
1. Compound (X) is and compound (Z) contains two 3C - 2e bonds
2. Compound (X) is and compound (Y) contains one 3C - 2e bonds
3. Compound (Y) is Lewis acid and stable due to back bonding
4. Compound (Z) reacts with excess of at high temperature gives inorganic graphite
The major products obtained in the reaction of oxalic acid with conc. upon heating are
1.
2.
3.
4.
reacts with
1. Only water
2. Only acids
3. Only alkalis
4. Both acids and alkalis
All the products formed in the oxidation of , are
1.
2.
3.
4.
In the structure of borax, the numbers of boron atoms and B-O-B units, respectively, are
1. 4 and 5
2. 4 and 3
3. 5 and 4
4. 5 and 3
Hydrolysis of gives X which on treatment with sodium carbonate produces Y. X and Y, respectively, are
1.
2.
3.
4.






