The cell reaction of micro electrochemical cell fromed during rusting of iron is:-
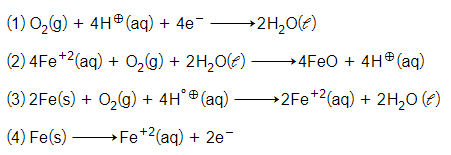
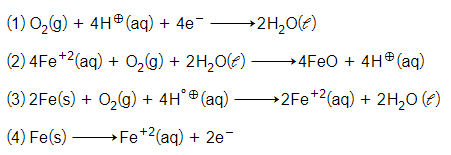
For the cell reaction
the change in free energy at a given temperature is a function of
(1) nC1
(2) n
(3) n (C1 +C2)
(4) nC2
The Emf of the following voltaic cell is:
\(\text {Ni }| \text {Ni}^{2+} (1 \text M) || \text {Au} ^{+3} \text {(1M) |Au}\)
\(\left ({~\mathrm{E}_{{\mathrm{Ni}^{+2}}/{\mathrm{Ni}}}^0=-0.25 \mathrm{~V}, \mathrm{E}_{{\mathrm{Au}^{+3}}/{\mathrm{Au}}}^0=1.50 \mathrm{~V}}\right )\)
1. 1.25 V
2. -1.75 V
3. 1.75 V
4. 4.0 V
The same amount of electric current is apassed through aqueous solution of MgSO4 and AlCl3. If 2.8 g Mg metal is deposited at amount of Al metal deposited in second cell will be
(1) 2.1 g
(2) 2.49 g
(3) 3.15 g
(4) 3.73g
What is the name of the device that converts the energy from the combustion of fuels such as hydrogen and methane directly into electrical energy?
(1) Fuel cell
(2) Electrolytic cell
(3) Dynamo
(4) Ni-Cd cell
gas can’t be obtained by the electrolysis of any salt because-
(A) Fluorine is the strongest reducing agent
(B) Fluorine is the strongest oxidising agent.
(C) Fluorine easily combine with atmospheric
(D) All
If the E°cell for a given reaction has a negative value then which of the following gives the correct relationships for the values of G° and Keq?
(a) G° < 0;Keq > 1
(b) G° < 0; Keq < 1
(c) G°> 0; Keq < 1
(d) G° > 0; Keq > 1
Acetic acid has Ka = 1.8 × 10–5 while formic acid had Ka = 2.1 × 10–4. What would be the magnitude of the emf of the cell
Pt(H2) Pt(H2) at 25°C
(1) 0.0315 volt
(2) 0.0629 volt
(3) 0.0455 volt
(4) 0.0545 volt
What will be the quantity of iron deposited by ferrous and ferric ion by 1F (Fe = 56)
(1) 14g, 9.3 g
(2) 28g, 18.6 g
(3) 56g, 37.2g
(4) None of these
The unit of specific conductance is:
| 1. | ohm-1 cm-1 | 2. | ohm cm |
| 3. | ohm cm-1 | 4. | ohm-1 cm |






