Consider the following metallurgical process:
Sulphide \(\xrightarrow[]{\Delta }\) oxide \(\xrightarrow[]{\Delta }\)
Impure metal \(\xrightarrow[]{\Delta }\) pure metal
The processes A, B, and C in the above mentioned reaction are, respectively:
1. Calcination, smelting, and electrolysis
2. Roasting, smelting, and electrolysis
3. Calcination, auto reduction, and bessemerisation
4. Roasting, aluminothermic reduction, electrolysis
Impure metal \(\xrightarrow[]{\Delta }\) pure metal
1. Calcination, smelting, and electrolysis
The depressant NaCN
1. Used to separate PbS from ZnS by forming a complex with PbS
2. Used to separate PbS from ZnS by forming a complex with ZnS
3. Used to form froth
3. Used as the collector
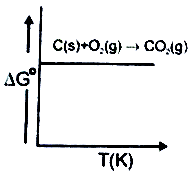
Ellingham diagram for the formation of is a straight line in the given graph. This is due to
1. Increase in entropy during formation
2. Decrease in entropy during formation
3. Entropy remains constant during formation
4. Cannot be predicted
Carbon cannot be used in the reduction of because
(I) It is non-metal
(2) The heat of the formation of is more than that of
(3) Pure carbon is not easily available
(4) The heat of formation of is too high
Which of the following statements is correct regarding the slag obtained during the extraction of a metal like copper or iron?
(1) The slag is lighter and has a lower melting point than the metal
(2) The slag is heavier and has a lower melting point than the metal
(3) The slag is lighter and has a higher melting point than the metal
(4) The slag is heavier and has a higher melting point than the metal
The process of bringing the metal ore into solution by the action of a suitable chemical reagent followed by extraction of the metal either by electrolysis or by a suitable precipitating agent is called-
1. Electrometallurgy
2. Hydrometallurgy
3. Electrorefining
4. Zone refining
Assertion : Sodium chloride is added during electrolysis of fused anhydrous magnesium
chloride.
Reason : Anhydrous magnesium chloride is obtained by heating hydrated magnesium
chloride, .
- If both the assertion and the reason are true and the reason is a correct explanation of the assertion
- If both the assertion and reason are true but the reason is not a correct explanation of the assertion
- If the assertion is true but the reason is false
- If both the assertion and reason are false
Silver containing lead as an impurity is removed by
1. Poling
2. Cupellation
3. Lavigation
4. Distillation
When an impurity in metal has a greater affinity for oxygen and is more easily oxidized than the metal itself, then the metal is refined by
(1) Cupellation
(2) Zone refining
(3) Poling
(4) Electrorefining
Consider the following reaction.
\(\small{Bauxite\ ore\ +\ NaOH\ (conc.) \xrightarrow[\Delta]{Digester} \underset{Soluble}{(A)}}\)
\((A) \xrightarrow{Fresh\ Al(OH)_3}\ \underset{White}{(B)}\ +\ (C)\)
\({(B)} \xrightarrow[]{\Delta}\ (D)\ +\ H_2O\)
What is the molecular formula of compound D?
1.
2.
3.
4.






