Which statement about the co-ordination number of a cation is true?
(1) Metal ions exhibit only a single characteristic co-ordination number
(2) The co-ordination number is equal to the number of ligands bonded to the metal atom
(3) The co-ordination number is determined solely by the number of empty d-orbitals in the atom
(4) Co-ordination number is equal to the number of coordinate bonds between the metal cation and ligands
If and both are present, both are complexed by and gas is passed, which one of the following pairs of complexes and their relative stability enables the separation of and ?
1. : more stable and : less stable
2. : less stable and : more stable
3. : more stable and : more stable
4. : less stable and : more stable
The spin-only magnetic moments of in Bohr Magnetons, respectively, are
1. 5.92 and 5.92
2. 4.89 and 1.73
3. 1.73 and 5.92
4. 1.73 and 1.73
The crystal field stabilization energies (CFSE) of high spin and low spin metal complexes in terms of , respectively are
1. -0.4 and -2.4
2. -2.4 and -0.4
3. -0.4 and 0.0
4. -2.4 and 0.0
The oxidation state of cobalt in the following molecule is
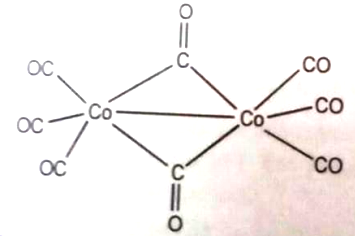
1. 3
2. 1
3. 2
4. 0
For a tetrahedral complex [MCl4]2-, the spin-only magnetic moment is 3.83 B.M. The element M is:
1. Co
2. Cu
3. Mn
4. Fe
Among the following, the species that is both tetrahedral and diamagnetic is
1.
2.
3.
4.
The number of ions produced in water by the dissolution of the complex having the empirical formula is
1. 1
2. 2
3. 4
4. 3
In aqueous solution, (X) reacts with molecular oxygen in the presence of excess liquor to give a new complex Y. The number of unpaired electrons in X and Y are, respectively
1. 3,1
2. 3,0
3. 3,3
4. 7,0
The number of geometrical isomers of , where en = ethylenediamine, is
1. 2
2. 3
3. 4
4. 1






