\(HC\equiv CNa \ + \ Cl-CH_{2}-CH_{2}-CH_{2}-I \ \rightarrow \ (A) \)
The major product (A) in the above reaction is :
1. H-CC-CH2-CH2-CH2-I
2. CH2=CH-CH2-I
3. H-CC-CH2-CH2-CH2-Cl
4. CH2=CH-CH2-Cl
\(HC\equiv CNa \ + \ Cl-CH_{2}-CH_{2}-CH_{2}-I \ \rightarrow \ (A) \)
The major product (A) in the above reaction is :
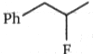
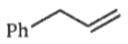
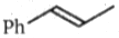
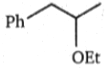
What is the major product (A) in the following reaction?

| 1. |  |
2. |  |
| 3. |  |
4. |  |
The major product of the following reaction is-
1.
2.
3.
4.
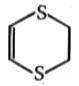
1-2-dichloro ethane + NaSCH2CH2SNa P (C4H8S2)
Unknown product (p) of the above reaction is:
1.
2. 
3.
4.
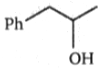
Which of the following alkyl halides has the maximum density?
| 1. | \(\mathrm{C}_3 \mathrm{H}_7 \mathrm{I} \) | 2. | \(\mathrm{C}_2 \mathrm{H}_5 \mathrm{I} \) |
| 3. | \(\mathrm{CH}_3 \mathrm{I} \) | 4. | \(\mathrm{CH}_3 \mathrm{Br}\) |
Grignard reagent shows addition on:
1. >C=O 2. -CN
3. >C=S 4. all of these
| 1. | They show H-bonding. | 2. | They are soluble in water. |
| 3. | They are soluble in organic solvents. | 4. | They do not contain any polar bond. |
Sodium ethoxide reacts with ethyl iodide to yield:
1. CH3CH3 2. C2H5OCH3
3. C2H5OC2H5 4. none of these
The set of reagents used to produce freon (CCl2F2) are:
| 1. | C + F2 + Cl2 → |
| 2. | CH3Cl + F2 → |
| 3. | \(CCl_4 + HF \ \xrightarrow[]{SbCl_5} \) |
| 4. | CCl4 + F2 → |