What is the structural difference between an aldehyde and a ketone?
1. -H-atom
2. H-atom combined with carbonyl group
3. OH and carbonyl group
4. None of the above
Ketones are less reactive than aldehydes because:
(a) C=O group is less polar in ketones
(b) of electromeric effect
(c) of steric hindrance to the attacking reagent
(d) none of the above
The important step in Cannizzaro's reaction is the intermolecular shift of:
(a) proton
(b) H-atom
(c) hydride ion
(d) hydronium ion
Pinacole is:
(a) 2,3-dimethyl-2,3-butandiol
(b) 3,3-dimethyl-2-propanone
(c) 3-methyl butan-2-ol
(d) none of the above
Benedict's solution provides:
(a) Ag+
(b) Cu2+
(c) Ba2+
(d) Li+
Acetaldehyde undergoes self condensation in presence of aluminium ethoxide to give ethyl acetate. This reaction is called:
(a) Perkin reaction
(b) Tischenko's reaction
(c) Cannizzaro's reaction
(d) Aldol condensation
An aldehyde which undergoes Cannizzaro's reaction and reduces Schiff's reagent but does not reduce Fehling's solution is:
(1) CH3CHO
(2) HCHO
(3) C6H5CHO
(4) salicylaldehyde
Which can be reduced to corresponding hydrocarbon by Zn/HCl ?
(a) Butan-2-one
(b) Acetic acid
(c) Acetamide
(d) Ethyl acetate
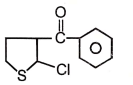
The IUPAC name of the is:
(1) 4-methyl isopropyl ketone
(2) 3-methyl-2-butanone
(3) isopropylmethyl ketone
(4) 2-methyl-3-butanone
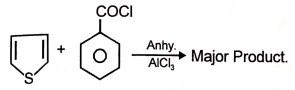
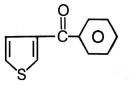
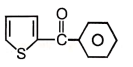
Major product maybe
1. 
2. 
3. 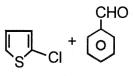
4.