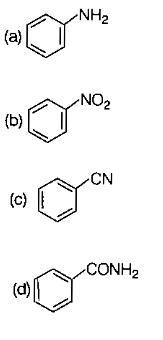
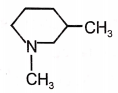
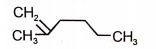
Which of the following compounds will give isocyanide test with ?
1. 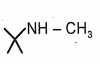
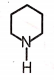
2. 
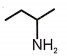
3. 
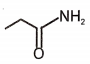
4. 
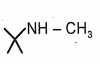



Give the incorrect order of basic strength
1.
2.
3.
4.
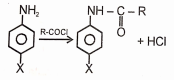
Which of the following substituent as 'X' not decreases the rate of reaction with respect to aniline?
1. 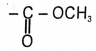
2.
3.
4. -CHO
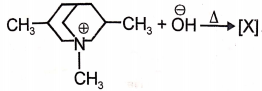
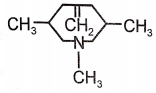
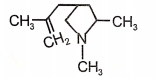
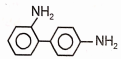
Identify the major product (A) formed in the following reaction/\.
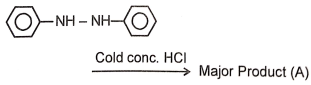
1. 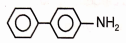 2.
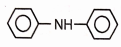
2. 
3. 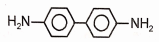 4.
4. 
Which of the following compounds will not give Hoffmann bromamide reaction?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Which of the following is more basic than aniline ?
1. Diphenylamine
2. Triphenylamine
3. p-nitroaniline
4. Benzylamine
Which of the following will be most stable diazonium salt ?
(a)
(b)
(c)
(d)
The correct statement regarding the basicity of arylamines is
(1) Arylamines are generally more basic than alkylamines because the nitrogen lone-pair electrons are not delocalized by interaction with the aromatic ring p-electron system.
(2) Arylamines are generally more basic than alkylamines because of aryl group.
(3) Arylamines are generally more basic than alkylamines, because the nitrogen atom in arylamines is sp-hybridized
(4) Arylamines are generally less basic than alkylamines because the nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring p-electron system.
A given nitrogen-containing aromatic compound a reacts with Sn/HCl, followed by HNO2 to give an unsatable compound B.B, on treament with phenol, forms a beautiful coloured compound C with the molecular formula C12H10N2O. The structure of compound A is