Zone refining is based on the principle that ___________.
1.
Impurities of low boiling metals can be separated by distillation.
2.
Impurities are more soluble in molten metal than in solid metal.
3.
Different components of a mixture are differently adsorbed on an adsorbent.
4.
Vapors of the volatile compounds can be decomposed in pure metal.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
In the metallurgy of aluminium ________________.
| 1. | Al3+ is oxidized to Al(s). |
| 2. | Graphite anode is oxidized to carbon monoxide and carbon dioxide. |
| 3. | Oxidation state of oxygen changes in the reaction at the anode. |
| 4. | Oxidation state of oxygen changes in the overall reaction involved in the process. |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Electrolytic refining is used to purify which of the following metals?
1. Cu and Zn
2. Ge and Si
3. Zr and Ti
4. Zn and Hg

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Answer the question on the basis of Figure
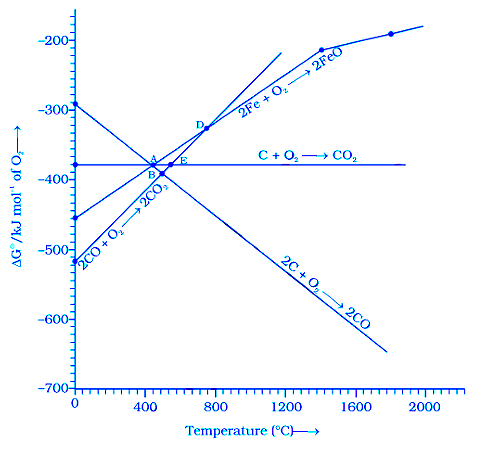
Choose the correct option of temperature at which carbon reduces FeO to iron and produces CO.
1. Below temperature at point A.
2. Approximately at the temperature corresponding to point A.
3. Above temperature at point A but below the temperature at point D.
4. Above temperature at point A.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Match the items of Column I with items of Column II and assign the correct code:
Column I Column II
(A) Pendulum (1) Chrome steel
(B) Malachite (2) Nickel steel
(C) Calamine (3)
(D) Cryolite (4)
(5)
1. A (1) B (2) C (3) D (4)
2. A (2) B (4) C (5) D (3)
3. A (2) B (3) C (4) D (5)
4. A (4) B (5) C (3) D (2)

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Match the items of Column I with the items of Column II and assign the correct
code :
| Column I | Column II | ||
| (A) | Colored bands | (1) | Zone refining |
| (B) | Impure metal to volatile complex | (2) | Fractional distillation |
| (C) | Purification of Ge and Si | (3) | Mond Process |
| (D) | Purification of mercury | (4) | Chromatography |
| (5) | Liquation | ||
1. A =(1); B=(2); C=(4); D=(5)
2. A=(4); B=(3); C=(1); D=(2)
3. A=(3); B=(4); C=(2); D=(1)
4. A=(5); B=(4); C=(3); D=(2)

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Match the items of Column I with the items of Column II and assign the correct code :
Column I Column II
(A) Sapphire (1)
(B) Sphalerite (2) NaCN
(C) Depressant (3) Co
(D) Corundum (4) ZnS
(5)
1. A (3) B (4) C (2) D (1)
2. A (5) B (4) C (3) D (2)
3. A (2) B (3) C (4) D (5)
4. A (1) B (2) C (3) D (4)

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Match the items of Column I with items of Column II and assign the correct
code :
| Column I | Column II | ||
| (A) | Blistered Cu | (i) | Aluminium |
| (B) | Blast furnace | (ii) | 2Cu2O + Cu2S → 6Cu + SO2 |
| (C) | Reverberatory furnace | (iii) | Iron |
| (D) | Hall-Heroult process | (iv) | FeO + SiO2 → FeSiO3 |
| (V) | 2Cu2S + 3O2 → 2Cu2O + 2SO2 | ||
| Options: | (a) | (b) | (c) | (d) |
| 1. | ii | iii | iv | i |
| 2. | i | ii | iii | v |
| 3. | v | iv | iii | ii |
| 4. | iv | v | iii | ii |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the following is not a mineral of aluminium?
1. Bauxite
2. Cryolite
3. Gibsite
4. Malachite

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Serpek’s method involves the heating of bauxite with
1. NaOH
2. Na2CO3
3. N2 +C
4. CaCO3






