The total energy of electron in the ground state of hydrogen atom is -13.6 eV. The kinetic energy of an electron in the first excited state is:
1. 3.4 eV
2. 6.8 eV
3. 13.6 eV
4. 1.7 eV

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
The ionisation potential of the hydrogen atom is \(13.6~\text{eV}.\) The hydrogen atoms in the ground state are excited by monochromatic radiation of photon energy of \(12.1~\text{eV}.\) According to Bohr’s theory, the spectral lines emitted by hydrogen atoms will be:
1. two
2. three
3. four
4. one

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
The total energy of an electron in an atom in an orbit is -3.4eV. Its kinetic and potential energies are, respectively:
1. 3.4 eV, 3.4 eV
2. -3.4 eV, -3.4 eV
3. -3.4 eV, -6.8 eV
4. 3.4 eV, -6.8 eV

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
Which statement about the Rutherford model of the atom is NOT true?
1. There is a positively charged center in an atom called the nucleus.
2. Nearly all the mass of an atom resides in the nucleus.
3. The size of the nucleus is the same as that of the atom.
4. Electrons occupy the space surrounding the nucleus.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
In 1911, the physician Ernest Rutherford discovered that atoms have a tiny, dense nucleus by shooting positively charged particles at a very thin gold foil. A key physical property which led Rutherford to use gold was that it was :
1. Electrically conducting
2. Highly malleable
3. Shiny
4. Non-reactive

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
Particles used in Rutherford's scattering experiment to deduce the structure of atoms :
1. Had atomic number 2 and were fully ionized
2. Had atomic number 2 and were neutral
3. Had atomic number 4 and were fully ionized
4. Had atomic number 4 and were neutral

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
The magnitude of the acceleration of the electron in the nth orbit of the hydrogen atom is and that of singly ionized helium atom is . The ratio is:
1. 1:8
2. 1:4
3. 1:2
4. Dependent on n

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
A hypothetical atom has 3 energy levels: the ground level, 2.00 eV, and 5.00 eV above the ground level. Which frequency of the spectral lines cannot be emitted when excited?
1.
2.
3.
4.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
Which of the following statements is inconsistent with the Bohr model of the atom?
1. Energy levels of the electron are stable and discrete.
2. An electron emits or absorbs radiation only when making a transition from one energy level to another.
3. To jump from a lower energy to a higher energy orbit, an electron must absorb a photon of precisely the right frequency such that the photon's energy equals the energy difference between the two orbits.
4. To jump from a higher energy to a lower energy orbit, an electron absorbs a photon of a frequency such that the photon's energy is exactly the energy difference between the two orbits.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.
The figure below illustrates an electron with an initial energy of -10 eV moving from point A to point B. What change accompanies the movement of the electron?
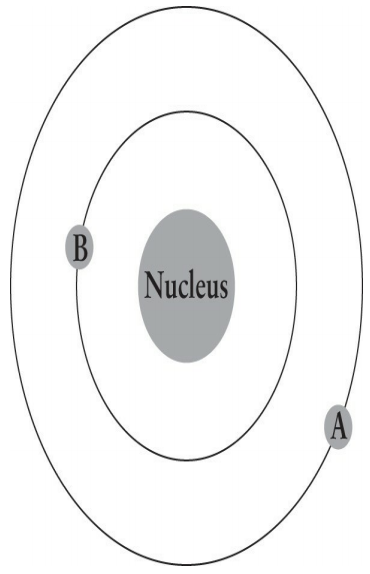
1. Absorption of a photon
2. Emission of a photon
3. The decrease in the atom's work function
4. Increase in the atom's total energy

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 14 chapters you need to be enrolled in MasterClass Course.






