ldentify the correct statement regarding entropy:
1. At absolute zero temperature, entropy of a perfectly crystalline
substance is +ve.
2. At absolute zero temperature, entropy of a perfectly crystalline
substance is taken to be zero
3. At absolute zero temperature, entropy of all crystalline substances
in taken to be 0.
4. At 0 C entropy of a perfectly crystalline substance is taken to
be 0.
The reaction that has a value of greater than zero among the following is-
\( \begin{align} & {{1}{.}\;{CaO}\left({s}\right){+}{CO}_{2}\rightarrow{CaCO}_{3}\left({s}\right)}\\ & {{2}{.}\;{NaCl}\left({aq}\right)\rightarrow{NaCl}\left({s}\right)}\\ & {{3}{.}\;{NaNO}_{3}\left({s}\right)\rightarrow{Na}^{{+}}\left({aq}\right){+}{NO}_{3}^{{-}}\left({aq}\right)}\\ & {{4}{.}\;{N}_{2}\left({g}\right){+}{3}{H}_{2}\left({g}\right)\rightarrow{2}{NH}_{3}\left({g}\right)} \end{align}\)
The net work done for an ideal gas is given as
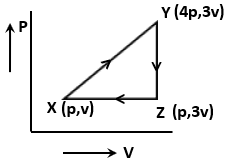
1. 3PV
2. 3PV
3. PV
4. Zero
Which of the following statements is correct for a spontaneous process?
1. The entropy of the system always increases.
2. The free energy of the system always increases.
3. The total entropy change is always negative.
4. The total entropy change is always positive.
Which one of the following has the maximum entropy of vaporization?
(Assuming all are in the liquid phase)
1. Water
2. Toluene
3. Diethyl ether
4. Acetone
Which of the following is not an endothermic reaction?
1. Combustion of methane
2. Decomposition of water
3. Dehydrogenation of ethane or ethylene
4. Conversion of graphite to diamond
Which of the following statements is false?
1. Work is a state function.
2. Temperature is a state function.
3. Density is a state function.
4. Entropy is state function.
Standard molar enthalpy of formation of is equal to
1. Zero
2. The standard molar enthalpy of combustion of gaseous carbon.
3. The sum of standard molar enthalpies of formation Of CO and
4. The standard molar enthalpy of combustion of carbon (graphite)
Which one of the following reactions obeys ∆ H ≠ ∆ E.?
| 1. | H 2 (g) + I 2 (g) ⇌ 2 H I(g) |
| 2. | HCl(aq) + NaOH (aq)→ NaCl(aq) + H2O(l) |
| 3. | C(s) + O2 (g) ⇌ CO2 (g) |
| 4. | N2 (g) + 3H2 (g) → 2NH3(g) |
The Gibbs free energy is defined as
1. G=HT.S
2. G=H+T.S
3. G=E T.S
4. G=E+ T.s






