Arrange the following compounds in increasing order of their boiling points:
CH3CHO, CH3CH2OH, CH3OCH3, CH3CH2CH3
The correct sequence of increasing orders of reactivity in nucleophilic addition reaction is:
1. Butanone < Propanone < Propanal < Ethanal
2. Butanone > Propanone > Propanal > Ethanal
3. Butanone < Propanal < Propanone < Ethanal
4. Propanal < Propanone < Ethanal < Butanone
Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions :Benzaldehyde, p-Tolualdehyde, p-Nitrobenzaldehyde, Acetophenone.
1. Acetophenone < Benzaldehyde < p-tolualdehyde < p-Nitrobenzaldehyde
2. Acetophenone < p-tolualdehyde < Benzaldehyde < p-Nitrobenzaldehyde
3. Acetophenone > p-tolualdehyde > Benzaldehyde < p-Nitrobenzaldehyde
4. Acetophenone < p-tolualdehyde < p-Nitrobenzaldehyde < Benzaldehyde
What will be the IUPAC names of the following compound ?
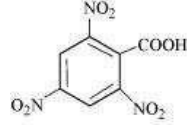
1. 2,4,6-Trinitrobenzoic acid
2. 1,3,5-Trinitrobenzoic acid
3. 2-oxo-1,3,5-dinitrobenzene
4. None of the above
Cannizzaro’s reaction is not given by:
| 1. |  |
2. |  |
| 3. | HCHO | 4. | CH3CHO |
The compound below is treated with a concentrated aqueous KOH solution. The products obtained are:

| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
What is the most suitable reagent for the below mentioned conversion?
\(\mathrm{CH_{3} — CH = CH — CH_{2} — \overset{\Large{O} \\~ ||}{C}—CH_{3} \rightarrow}\)
\(\mathrm{CH_{3} — CH = CH — CH_{2} — \overset{\Large{O} \\~ ||}{C}—OH}\)
1. Tollens' reagent
2. Benzoyl peroxide
3. \(\mathrm I_{2}\) and NaOH solution
4. Sn and NaOH solution
Match the common names given in Column I with the IUPAC names given in Column II.
| Column l (Common names) |
Column ll (IUPAC names) |
| A. Cinnamaldehyde | 1. Pentanal |
| B. Acetophenone | 2. Prop-2-enal |
| C. Valeraldehyde | 3. 1-Phenylethanone |
| D. Acrolein | 4. 3-Phenylprop-2-en-al |
Codes:
| A | B | C | D | |
| 1. | 2 | 3 | 4 | 1 |
| 2. | 3 | 1 | 4 | 2 |
| 3. | 1 | 4 | 3 | 2 |
| 4. | 4 | 3 | 1 | 2 |
Match the acids given in Column I with their correct IUPAC names given in Column II and mark the appropriate option:
| Column l (Acids) |
Column ll (IUPAC names) |
| A. Phthalic acid | 1. Hexane-1,6-dioic acid |
| B. Glutaric acid | 2. Benzene-1,2-dicarboxylic acid |
| C. Succinic acid | 3. Pentane-1,5-dioic acid |
| D. Adipic acid | 4. Butane-1,4-dioic acid |
Codes:
| A | B | C | D | |
| 1. | 2 | 3 | 4 | 1 |
| 2. | 3 | 1 | 4 | 2 |
| 3. | 1 | 4 | 3 | 2 |
| 4. | 4 | 3 | 2 | 1 |
Match the reactions given in Column-I with the suitable reagents given in Column-II.
| Column-l (Reactions) |
Column-ll
(Reagents)
|
||
| A. | Benzophenone → Diphenylmethane | I. | LiAlH4 |
| B. | Benzaldehyde → 1-Phenylethanol | II. | DlBAL-H |
| C. | Cyclohexanone → Cyclohexanol | III. | Zn(Hg)/Conc. HCl |
| D. | Phenyl benzoate → Benzaldehyde | IV. | CH3MgBr |
Codes:
| A | B | C | D | |
| 1. | II | III | IV | I |
| 2. | III | IV | I | II |
| 3. | I | IV | III | II |
| 4. | IV | III | II | I |






