The axial bonds are longer as compared to equatorial bonds in PCl5 because:
1.
Axial bond pairs suffer more repulsion from the equatorial bond pairs
2.
Equatorial bond pairs suffer more repulsion from the axial bond pairs
3.
Both 1, and 2
4.
None of the above
The relation between hydrogen bond and the van der Waals forces is:
| 1. | Both the bonds have equal strength. |
| 2. | Hydrogen bonds are weaker than van der Waals forces. |
| 3. | Hydrogen bonds are stronger than van der Waals forces. |
| 4. | None of the above |
If the main axis of a diatomic molecule is z, then the molecular orbital px and py overlap to form which of the following orbital:
1. molecular orbtial
2. σ molecular orbital
3. δ molecular orbtial
4. No bond will form
The significance of the octet rule is:
1. To determine the stability of atoms.
2. To find the hybridization of elements.
3. To calculate the number of lone pairs.
4. None of the above.
The incorrect statement among the following is:
| 1. | The sigma bond forms via head-on overlap and the pie bond forms via sidewise overlapping of orbitals. |
| 2. | s and p orbitals combine to form a sigma bond as well as a pie bond. |
| 3. | Hybrid orbitals form sigma bonds only. |
| 4. | Sigma bonds are stronger than pie bonds. |
The correct Lewis structure of acetic acid is:
| 1. |  |
2. |  |
| 3. |  |
4. | None of the above. |
H2 molecule on the basis of valence bond theory is formed due to:
1. Attractive forces being more than the repulsive forces.
2. Attractive forces being less than the repulsive forces.
3. No force of attraction.
4. Only repulsive force.
Hybridization of C1 and C2 in the structure given below are
1.
2.
3.
4.
Consider the following structure.
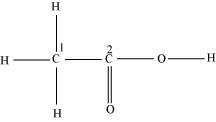
The hybridization of C1, and C2 carbon in the structure are -
1. = , =
2. = , =
3. = , =
4. None of the above
The number of resonating structures exhibited by \(CO^{2-}_3\) ion are :
| 1. | 2 | 2. | 3 |
| 3. | 4 | 4. | 5 |







