The correct number of σ and π bonds in the molecule is:
σ (C - H)
σ (C - N)
σ (N - O)
π (N=O)
1.
1
1
1
3
2.
1
1
3
1
3.
1
3
1
3
4.
3
1
1
1
Subtopic: Hybridisation & Structure of Carbon Compounds |
91%
Level 1: 80%+
Hints
The correct IUPAC name is -
| 1. | 2,2-Dimethylpentane | 2. | 2-Dimethylpentane |
| 3. | 1-Dimethylpentane | 4. | 2,2-Dimethylethane |
Subtopic: Nomenclature |
89%
Level 1: 80%+
Hints
The correct IUPAC name of the following structure is

| 1. | 2,4,7-Trimethyloctane | 2. | 2,5,7-Trimethyloctane |
| 3. | 2,3,7-Trimethyloctane | 4. | All of the above |
Subtopic: Nomenclature |
88%
Level 1: 80%+
Hints
The IUPAC name of the following molecule is -

| 1. | 2-Chloro-4-methylpentane | 2. | 4-Chloro-2-methylpentane |
| 3. | 4-Chloro-4-methylpentane | 4. | 2-Chloro-2-methylpentane |
Subtopic: Nomenclature |
87%
Level 1: 80%+
Hints
The species present below is
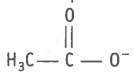
1. carbocation
2. electrophile
3. nucleophile
4. carboanion
Subtopic: Nucleophile & Electrophile |
71%
Level 2: 60%+
Hints
ion is a-
1. carbocation
2. nucleophile
3. electrophile
4. carboanion
Subtopic: Nucleophile & Electrophile |
82%
Level 1: 80%+
Hints
ion is a -
1. carbocation
2. carboanion
3. electrophile
4. nucleophile
Subtopic: Nucleophile & Electrophile |
79%
Level 2: 60%+
Hints
molecule is -
1. carbocation
2. nucleophile
3. electrophile
4. carboanion
Subtopic: Nucleophile & Electrophile |
70%
Level 2: 60%+
Hints
The species present below is
1. nucleophile
2. carbocation
3. electrophile
4. carboanion
Subtopic: Nucleophile & Electrophile |
82%
Level 1: 80%+
Hints
Chlorine atom can be classified as:
| 1. | Carbocation | 2. | Nucleophile |
| 3. | Electrophile | 4. | Carbanion |
Subtopic: Nucleophile & Electrophile |
Level 3: 35%-60%
Hints
Links






