Select Question Set:
Hydrogen gas is contained in a vessel and the RMS speed of the gas molecules is \(v\). The gas is heated isobarically so that its volume doubles, then it is compressed isothermally so that it returns to the same volume. The final RMS speed of the molecules will be:
1.
2\(v\)
2.
\(v\)/2
3.
\(v\)\(\sqrt2\)
4.
\(v\)/\(\sqrt2\)
Subtopic: Types of Velocities |
72%
Level 2: 60%+
Hints
Which of the following graphs correctly represents the variation of \(\beta=-\dfrac{1}{V}\dfrac{dV}{dp} \) with \(p \) for an ideal gas at constant temperature?
| 1. | 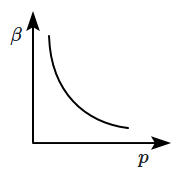 |
2. | 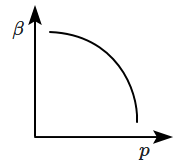 |
| 3. | 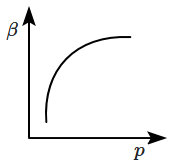 |
4. | 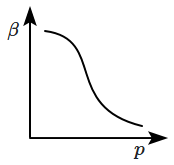 |
Subtopic: Ideal Gas Equation |
74%
Level 2: 60%+
Please attempt this question first.
Hints
Please attempt this question first.
If the pressure in a closed vessel is reduced by removing some of the gas, how is the mean free path between two gas molecules affected?
| 1. | It increases. |
| 2. | It decreases. |
| 3. | It remains unchanged. |
| 4. | It increases or decreases depending on the nature of the gas. |
Subtopic: Mean Free Path |
64%
Level 2: 60%+
Please attempt this question first.
Hints
Please attempt this question first.
Suppose that the average kinetic energy (translational & rotational) of random molecular motion of helium \(\mathrm{(He})\) at temperature \(T_\mathrm{He}\) is equal to that of hydrogen \(\mathrm{(H_2})\) at temperature \(T_\mathrm{H_2}.\) Then;
| 1. | \(T_\mathrm {H_{2}}=T_\mathrm{H e}\) | 2. | \(\dfrac{T_\mathrm{H_2}}{2}=\dfrac{T_\mathrm{He}}{4}\) |
| 3. | \(5 T_\mathrm{H_2}=3 T_\mathrm{He}\) | 4. | \(\dfrac{T_\mathrm{H_{2}}}{5}=\dfrac{T_\mathrm{{He }}}{3}\) |
Subtopic: Kinetic Energy of an Ideal Gas |
55%
Level 3: 35%-60%
Hints
Which of the following parameters is the same for molecules of all gases at a given temperature?
1. mass
2. speed
3. momentum
4. kinetic energy
Subtopic: Kinetic Energy of an Ideal Gas |
69%
Level 2: 60%+
Hints
The volume occupied by the molecules contained in \(4.5~\text{kg}\) water at STP, if the molecular forces vanish away, is:
| 1. | \(5.6~\text{m}^3\) | 2. | \(5.6\times10^{6}~\text{m}^3\) |
| 3. | \(5.6\times10^{3}~\text{m}^3\) | 4. | \(5.6\times10^{-3}~\text{m}^3\) |
Subtopic: Ideal Gas Equation |
Level 3: 35%-60%
NEET - 2022
Hints
Given below are two statements:
| Assertion (A): | The molecules of a monoatomic gas has three degrees of freedom. |
| Reason (R): | The molecules of diatomic gas have five degrees of freedom. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | Both (A) and (R) are False. |
Subtopic: Law of Equipartition of Energy |
92%
Level 1: 80%+
Hints
Given below are two statements:
| Assertion (A): | The molar heat capacity of the gas can have any value from \(-\infty\) to \(\infty\). |
| Reason (R): | The molar heat capacity of the gas for the isothermal process is \(\infty\). |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | Both (A) and (R) are False. |
Subtopic: Specific Heat |
64%
Level 2: 60%+
Hints
A gas has \(n\) degrees of freedom. The ratio of the specific heat of the gas at constant volume to the specific heat of the gas at constant pressure will be:
| 1. | \({\dfrac{n} {n+2}}\) | 2. | \({\dfrac{n+2} {n}}\) |
| 3. | \({\dfrac{n} {2n+2}}\) | 4. | \({\dfrac{n} {n-2}}\) |
Subtopic: Specific Heat |
71%
Level 2: 60%+
JEE
Please attempt this question first.
Hints
Please attempt this question first.
A vessel contains \(16\) g of hydrogen and \(128\) g of oxygen at standard temperature and pressure. The volume of the vessel in cm3 is:
1. \(72\times10^{5}\)
2. \(32\times10^{5}\)
3. \(27\times10^{4}\)
4. \(54\times10^{4}\)
1. \(72\times10^{5}\)
2. \(32\times10^{5}\)
3. \(27\times10^{4}\)
4. \(54\times10^{4}\)
Subtopic: Ideal Gas Equation |
74%
Level 2: 60%+
JEE
Please attempt this question first.
Hints
Please attempt this question first.
Select Question Set:






