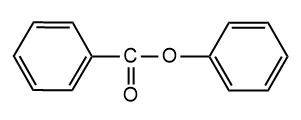
 and
and 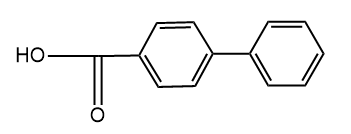 are
are
1. Position isomers
2. Chain isomers
3. Functional isomers
4. Metamers
 and
and  are
areWhich of the following statements about the inductive effect is correct?
| 1. | The inductive effect transfers electrons from one carbon atom to another. |
| 2. | The inductive effect operates in both \(\sigma\)- and \(\pi\)-bonds. |
| 3. | The inductive effect does not create any charge in the molecule. |
| 4. | The inductive effect creates partial charges and is distance-dependent. |
The correct stability order of following species is :
(1) x > Y > w > z
(2) y > x > w > z
(3) x > w > z > y
(4) z > x > y > w
Acid strength of the conjugate acids of the following are-
(1) I > II > III > IV
(2) III > II > I > IV
(3) IV > III > II > I
(4) None of these
The correct acidic strength order of acidic hydrogen x, y and z is respectively.
(1) x > z > y
(2) x > y > z
(3) z > y > x
(4) y > z > x
The correct basic strength order is:
(1) I > II > IV > III
(2) IV > III > II > I
(3) III > II > IV > I
(4) III > IV > II > I
The Prussian blue colour obtained during the test of nitrogen by Lassaigne's test is due to the formation of-
| 1. | Fe4[Fe(CN)6]3 | 2. | Na3[Fe(CN)6] |
| 3. | Fe(CN)3 | 4. | Na4[Fe(CN)5NOS] |
The IUPAC name of the below given compound is:
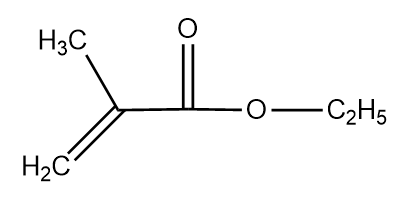
1. Ethyl 2-methylprop-2-enoate
2. Ethyl 2-methylprop-1-enoate
3. 1-Ethoxy 2-methylprop-2-enoate
4. 1-Ethoxy 2-methylprop-2-enal
The IUPAC name of the given compound is:
1. 2-Phenylpropan-3-al
2. Formylethylbenzene
3. 2-Phenylpropanal
4. Ethylformylbenzene
The IUPAC name of the given compound is:
1. Ethanoic propanoic anhydride
2. Propanoic ethanoic anhydride
3. 1-Ethanoyloxypropanone
4. 3-Ethanoyloxypropan-3-one












