Conversion of cyclohexene to cyclohexanol can be conveniently achieved by:
1. NaOH + H2O
2. Br2-H2O
3. Hydroboration, oxidation
4. Hydroboration hydrolysis
1. NaOH + H2O
Bromination of (E)-2-butenedioic acid gives
(1) (2R, 3S)-2, 3-dibromosuccinic acid
(2) (2R, 3R)-2, 3-dibroniosuccinic acid
(3) a mixture of (2R, 3R) and (2S, 3S)-2, 3-dibromosuccinic acid
(4) (2S, 3S)-2, 3-dibromosuccinic acid
Consider the following reaction:-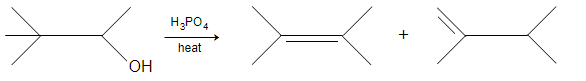
Which response contains all the correct statement about this process?
(1)Dehydration
(2)E2 mechanism
(3)Carbon skeleton migration
(4)Most stable alkene will form
(5)Single-step reaction
(a)1,3 (b)1,2,3 (c)1,2,5 (d)1,3,4

(1) cis-2-butene
(2) trans-2-butene
(3) 1-butene
(4) Iso-butene
Which position will be attacked most rapidly by the nitronium ion (-NO2)+ when the compound undergoes nitration with HNO3/H2SO4 :-
1. A
2. B
3. C
4. D
The electrophilic aromatic substitution proceeds through a:
(1) free radical
(2) sigma complex
(3) benzyne
(4) carbene
Which of the following substitution of benzene is ortho-para in electrophilic substitution and ortho-para in nucleophilic substitution ?
(1) -NO2
(2) -NO
(3) -SO3H
(4) -SO2Me
Which one of the following undergoes nucleophilic aromatic substitution at the fastest rate?
(1)
(2)
(3)
(4)
Many organic compounds are prepared by using PCl5 because:
(1) OH group of alcohol is easily replaced by chlorine atom
(2) chlorines are added to the unsaturated compounds
(3) it removes water from organic compounds
(4) phosphorus atoms are entered in the alcohol
identify the product in the given reaction:
1.
2.
3.
4.











