An unknown alcohol is treated with the “Lucas reagent” to determine whether the alcohol is primary, secondary or tertiary. Which alcohol reacts the fastest and by what mechanism:
1. secondary alcohol by SN2
2. tertiary alcohol by SN2
3. secondary alcohol by SN1
4. tertiary alcohol by SN1
Sodium phenoxide when heated with CO2 under pressure at 125°C yields a product which on acetylation produces C.
The major product C would be :
(1)
(2)
(3)
(4)
A compound with molecular formula C4H10O3 is converted by the action of acetyl chloride to a compound with molar mass 190. The original compound has:
(1) one OH group
(2) two OH groups
(3) three OH groups
(4) no OH group
Phenyl ethyl ether with concentrated hydrobromic acid on boiling yields:-
1. Phenol and ethyl bromide
2. Bromobenzene and ethanol
3. Phenol and ethane
4. Bromobenzene and ethane
An organic compound A reacts with sodium metal and forms B. On heating with conc. H2SO4, A gives diethyl ether. So A and B are:
1. C3H7OH and CH3ONa
2. CH3OH and CH3ONa
3. C4H9OH and C4H9ONa
4. C2H5OH and C2H5ONa
Which will form two oximes with NH2OH?
(1) CH3COCH3
(2) CH3CH2COCH3
(3) CH3CH2COCH2CH3
(4)
Identify A, X, Y and Z
(1) A-methoxymethane, X-ethanoic acid, Y-acetate ion, Z-hydrazine
(2) A- methoxymethane, X-ethanol, Y-ethanoic acid, Z- semicarbazide
(3) A-Acetaldehyde, X-ethanol, Y-but-2-en-1-ol, Z- semicarbazone
(4) A-ethanol, X-acetaldehyde, Y-butanone, Z-hydrazone
Reaction of phenol with chloroform in the presence of dilute sodium hydroxide finally
introduces, which one of the following functional group?
1. -CH2Cl
2. -COOH
3. -CHCl2
4. -CHO
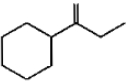
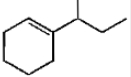
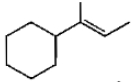
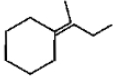
Which of the following is not the product of dehydration of
1. 
2. 
3. 
4. 
H2COH.CH2OH on heating with periodic acid gives :-
1. 2CO2
2. 2HCOOH
3. CHO-CHO
4.














