Which one of the following pairs of species have the same bond order?
(1) CO, NO
(2) O2- , CN-
(3) CN- , NO+
(4) N+ , CN+
In which of the following compounds is an intramolecular hydrogen bond present?
| 1. | H2O2 | 2. | HCN |
| 3. | Cellulose | 4. | Concentrated acetic acid |
In which of the following molecules, all atoms are coplanar ?
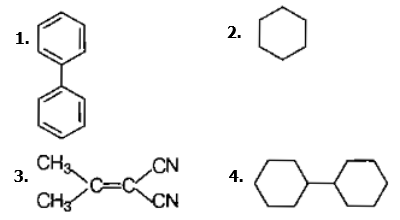
Predict the correct order among the following.
(1) lone pair-lone pair>bond pair-bond pair>lone pair-bond pair
(2) bond pair-bond pair>lone pair-bond pair>lone pair-lone pair
(3) lone pair-bond pair>bond pair-bond pair>lone pair-lone pair
(4) lone pair-lone pair>lone pair-bond pair>bond pair-bond pair
The pair of electrons in the given carbanion, CH3C≡C-, is present in which of the following orbitals?
| 1. | sp3 | 2. | sp2 |
| 3. | sp | 4. | 2p |
The incorrect statement among the following regarding the molecules of CH4, NH3, and H2O is:
| 1. | The H-O-H bond angle in H2O is larger than the H-C-H bond angle in CH4. |
| 2. | The H-O-H bond angle in H2O is smaller than the H-N-H bond angle in NH3. |
| 3. | The H-C-H bond angle in CH4 is larger than the H-N-H bond angle in NH3. |
| 4. | The H-C-H bond angle in CH4, the H-N-H bond angle in NH3, and the H-O-H bond angle in H2O are all greater than 90°. |
The species, having bond angles of 120° is :
1. PH3
2. ClF3
3. NCl3
4. BCl3
Which of the following species has a bond angle of \(120^\circ\)?
1. \(\mathrm{{SF}_6}\)
2. \(\mathrm{NCl_3}\)
3. \(\mathrm{BCl_3}\)
4. \(\mathrm{PH_3}\)
The hybridisations of atomic orbitals of nitrogen in \(\mathrm{NO}^{+}\), \(N O_3^{-}\) and NH3 respectively are:
1.
2.
3.
4.
Pair of ions among the following that are isoelectronic and isostructural:
1.
2.
3.
4.






