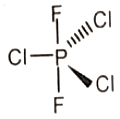
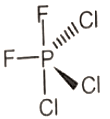
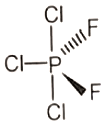
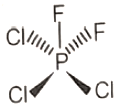
Which of the following molecules has no dipole moment?
1.
2.
3.
4.
The element which readily forms an ionic bond has the electronic configuration
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The order of electronegativity of carbon in sp, hybridized states follows:
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Among the following atomic orbital overlaps, the non-bonding overlap is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The compounds containing sp hybridized carbon atoms are:
| i. |  |
ii. |  |
| iii. | iv. |
1. i and ii
2. iii and iv
3. ii and iii
4. i and iv

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The hybridization of the central atom and the shape of ion, respectively, are
1.
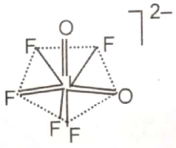
2.
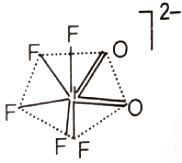
3.
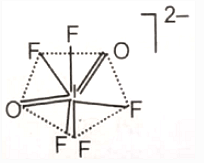
4.
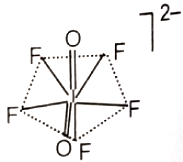
Which of the following contain a maximum number of electrons in the antibonding molecular orbitals?
| 1. | \(O^{2-}_2\) | 2. | \(O_2\) |
| 3. | \(O^{-}_2\) | 4. | \(O^{+}_2\) |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the following compounds has the least tendency to form hydrogen bonds between molecules?
1.
2.
3. HF
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.