Compound A, is found to react with NaOI (produced by reacting Y with NaOH) and yields a yellow precipitate with characteristic smell.
A and Y are respectively
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Carboxylic acids have higher boiling points than aldehydes, ketones and even alcohols of comparable molecular mass. It is due to their
1. Formation of intramolecular H-bonding
2. Formation of carboxylate ion
3. More extensive association of carboxylic acid via van der waals force of attraction
4. Formation of intermolecular H-bonding

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Of the following, which is the product formed when cyclohexanone undergoes aldol condensation followed by heating?
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Consider the reactions:

Identify A, X, Y and Z
1. A- Methoxymethane, X-Ethanol, Y-Ethanoic acid, Z-Semicarbazide.
2. A- Ethanal, X-Ethanol, Y-But-2-enal, Z-Semicarbazide.
3. A- Ethanol, X-Acetaldehyde, Y-Butanone, Z-Hydrazone.
4. A- Methoxymethane, X-Ethanoic acid, Y-Acetate, Z-Hydrazine.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the following reactions is appropriate for converting acetamide to methanamine?
1. Hoffmann hypobromamide reaction
2. Stephens reaction
3. Gabriels phthalimide synthesis
4. Carbylamine reaction

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
CH2=CH-CHO+HCNA(Major),
the compound 'A' is
1. CH2=CH-CH-CH(OH)(CN)
2. CH3-CH(CN)-CHO
3. CH2(CN)-CH2-CHO
4. CH3-C(OH)(CN)-CH3
The slowest step of Cannizarro's reaction is
1. Attack of nucleophilic
2. Hydride shift
3. Formation of anion
4. Transfer of proton

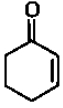












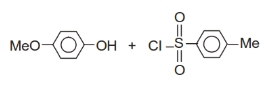



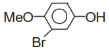


 +Br2 ?
+Br2 ?


