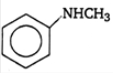
An amide (A) C3H7NO on hydrolysis gives an amine
(B) and an acid (C). B gives carbylamine reaction
where as C reduces Tollen’s reagent. (A) is
1. CH3CONHCH3
2. CH3CH2CONO2
3. HCONHC2H5
4.
(B) and an acid (C). B gives carbylamine reaction
where as C reduces Tollen’s reagent. (A) is
The amine that reacts with Hinsberg's reagent to give an alkali insoluble product is:-
1.
2.
3.
4.
The correct order of the basic strength of methyl substituted amines in aqueous solution is:
1.
2.
3.
4.
Which of the following is more basic than aniline?
1. Diphenylamine
2. Triphenylamine
3. p-nitroaniline
4. Benzylamine
Which of the following compounds, when reduced with lithium aluminium hydride, produces a secondary amine?
1. Nitroethane
2. Methylisocyanide
3. Acetamide
4. Methyl cyanide

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the following statements about primary amines is false?
1. Alkyl amines are stronger bases than aryl amines
2. Alkyl amines react with, nitrous acid to produce alcohols
3. Aryl amines react with nitrous acid to produce phenols
4. Alkyl amines are stronger bases than ammonia

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
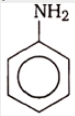
The structure of 'Y' would be





To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
What is the product obtained in the following reaction?
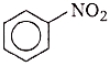
1.
2.
3.
4.
2. 1, 4– Dinitrobenzene
3. 1, 2, 4– Trinitobenzene
4. 1, 2– Dinitrobenzene

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.