Which of the following statements about primary amines is false?
1. Alkyl amines are stronger bases than aryl amines
2. Alkyl amines react with, nitrous acid to produce alcohols
3. Aryl amines react with nitrous acid to produce phenols
4. Alkyl amines are stronger bases than ammonia

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
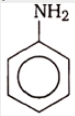
The structure of 'Y' would be





To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
What is the product obtained in the following reaction?
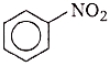
1.
2.
3.
4.
2. 1, 4– Dinitrobenzene
3. 1, 2, 4– Trinitobenzene
4. 1, 2– Dinitrobenzene

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
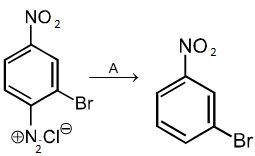
2. H3PO2 and H2O
3. H+ / H2O
4. HgSO4 / H2SO4

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Among the following options, which diazonium salt would be the most stable?
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
 +
+ 


To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
2. Azoxybenzene
3. Azobenzene
4. Aniline
Method by which aniline cannot be prepared is
1. Hydrolysis phenyl isocyanide with acidic solution
2. Degradation of benzamide with bromine in alkaline Solution
3. Reduction of nitrobenzene with H/Pd in ethanol
4. Potassium salt of phthalimide treated with chlorobenzene followed by the hydrolysis aqueous NaOH solution

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
| 1. | Arylamines are generally more basic than alkylamines because the nitrogen lone-pair electrons are not delocalized by interaction with the aromatic ring -electron system. |
| 2. | Arylamines are generally more basic than alkylamines because of aryl group. |
| 3. | Arylamines are generally more basic than alkylamines because the nitrogen atom in arylamines is sp-hybridized. |
| 4. | Arylamines are generally less basic than alkylamines because the nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring -electron system. |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.










