The cell potential for this reaction is 0.46V. Which of the following change will increase the potential the most ?
(1) concentration is doubled
(2) [Cu2+] concentration is halved
(3) Size of Cu(s) electrode is doubled
(4) size of Ag(s) electrode is decreased to half
What will be the value of for the rection
if
(1) -65.62 KJ
(2) -75.27 KJ
(3) -150.54 KJ
(4) +150.54 KJ
The cell reaction of micro electrochemical cell fromed during rusting of iron is:-
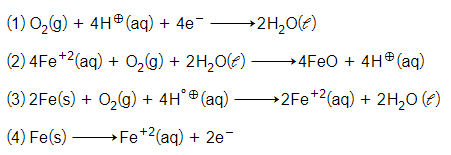
For the cell reaction
the change in free energy at a given temperature is a function of
(1) nC1
(2) n
(3) n (C1 +C2)
(4) nC2
The Emf of the following voltaic cell is:
\(\text {Ni }| \text {Ni}^{2+} (1 \text M) || \text {Au} ^{+3} \text {(1M) |Au}\)
\(\left ({~\mathrm{E}_{{\mathrm{Ni}^{+2}}/{\mathrm{Ni}}}^0=-0.25 \mathrm{~V}, \mathrm{E}_{{\mathrm{Au}^{+3}}/{\mathrm{Au}}}^0=1.50 \mathrm{~V}}\right )\)
1. 1.25 V
2. -1.75 V
3. 1.75 V
4. 4.0 V
The same amount of electric current is apassed through aqueous solution of MgSO4 and AlCl3. If 2.8 g Mg metal is deposited at amount of Al metal deposited in second cell will be
(1) 2.1 g
(2) 2.49 g
(3) 3.15 g
(4) 3.73g
A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known as
(1) fuel cell
(2) electrolytic cell
(3) dynamo
(4) Ni-Cd cell
gas can’t be obtained by the electrolysis of any salt because-
(A) Fluorine is the strongest reducing agent
(B) Fluorine is the strongest oxidising agent.
(C) Fluorine easily combine with atmospheric
(D) All
If the E°cell for a given reaction has a negative value then which of the following gives the correct relationships for the values of G° and Keq?
(a) G° < 0;Keq > 1
(b) G° < 0; Keq < 1
(c) G°> 0; Keq < 1
(d) G° > 0; Keq > 1
Acetic acid has Ka = 1.8 × 10–5 while formic acid had Ka = 2.1 × 10–4. What would be the magnitude of the emf of the cell
Pt(H2) Pt(H2) at 25°C
(1) 0.0315 volt
(2) 0.0629 volt
(3) 0.0455 volt
(4) 0.0545 volt






