The number of σ and π bonds in the molecule are:
1. 7 = ( , 11 = , and 0= π
2. 6 = ( , 12 = , and 0= π
3. 12 = ( , 6 = , and 1= π
4. 5 = ( , 13 = , and 1= π
Indicate the σ and π bonds in this molecule
1. two C–C sigma ( bonds, two C–H sigma bonds, and one C=C pi bonds
2. four C–C sigma ( bonds, four C–H sigma bonds, and two C=C pi bonds
3. two C–C sigma ( bonds, four C–H sigma bonds, and two C=C pi bonds
4. two C–C sigma ( bonds, two C–H sigma bonds, and two C=C pi bonds
The correct number of σ and π bonds in the molecule is:
| σ (C - H) | σ (C - N) | σ (N - O) | π (N=O) | |
| 1. | 1 | 1 | 1 | 3 |
| 2. | 1 | 1 | 3 | 1 |
| 3. | 1 | 3 | 1 | 3 |
| 4. | 3 | 1 | 1 | 1 |
The correct IUPAC name is -
| 1. | 2,2-Dimethylpentane | 2. | 2-Dimethylpentane |
| 3. | 1-Dimethylpentane | 4. | 2,2-Dimethylethane |
The correct IUPAC name of the following structure is

| 1. | 2,4,7-Trimethyloctane | 2. | 2,5,7-Trimethyloctane |
| 3. | 2,3,7-Trimethyloctane | 4. | All of the above |
The IUPAC name of the following molecule is -

| 1. | 2-Chloro-4-methylpentane | 2. | 4-Chloro-2-methylpentane |
| 3. | 4-Chloro-4-methylpentane | 4. | 2-Chloro-2-methylpentane |
The species present below is
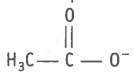
1. carbocation
2. electrophile
3. nucleophile
4. carboanion
ion is a-
1. carbocation
2. nucleophile
3. electrophile
4. carboanion
ion is a -
1. carbocation
2. carboanion
3. electrophile
4. nucleophile
molecule is -
1. carbocation
2. nucleophile
3. electrophile
4. carboanion






