n-Butylbenzene on oxidation with hot alkaline KMnO4 gives:
1.
Benzoic acid
2.
Butanoic acid
3.
Benzyl alcohol
4.
Benzaldehyde
Which of the following compounds undergoes bromination of its aromatic ring (electrophilic aromatic substitution) at the fastest rate?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Consider the following sequence of reactions.
\(\mathrm{C}_6 \mathrm{H}_6+\mathrm{CH}_3 \mathrm{CH}=\mathrm{CH}_2 \xrightarrow[\text { heat }]{\mathrm{H}_3 \mathrm{PO}_4} \mathrm{~A} \xrightarrow[2.~ \mathrm{H}_3 \mathrm{O}^{+}, \text {Heat }]{1. ~\mathrm{O}_2 \text { Heat }} \mathrm{B}+\mathrm{C}\)
The products (B) and (C) are respectively:
1. Benzaldehyde, and acetaldehyde
2. Benzoic acid, and acetic acid
3. Phenol, and propionaldehyde
4. Phenol, and acetone
Consider the following sequence of reactions.
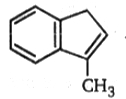 AB. The final product (B) is:
AB. The final product (B) is:
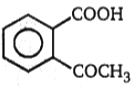
1. 
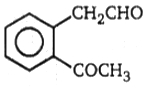
2. 
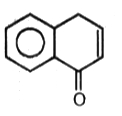
3. 
4. 

1.
2. Cl-
3. Cl
4. Cl2+
The major product expected from the mono-bromination of phenyl benzoate is:
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
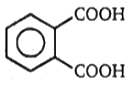
(A) C8H10 (B) C8H6O4 C8H5BrO4 (C) (one-product only)
The structure of A in the above-mentioned reaction is-
| 1. |  |
2. |  |
| 3. |  |
4. |  |
, unknown reagent (c) is:
1. LiALH4
2. NaBH4
3. LiAlH4(t-BuO)3
4. PCC/CH2CL2

The product (B) in the above-mentioned reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |

(a)
(b)
(c)
(d)










