Unlock IMPORTANT QUESTION
This question was bookmarked by 5 NEET 2025 toppers during their NEETprep journey. Get Target Batch to see this question.
✨ Perfect for quick revision & accuracy boost
Buy Target Batch
Access all premium questions instantly
Consider the following sequence of reactions.
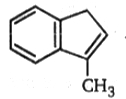 AB. The final product (B) is:
AB. The final product (B) is:
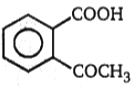
1. 
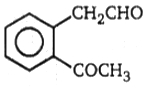
2. 
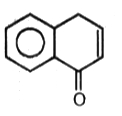
3. 
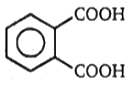
4. 
 AB. The final product (B) is:
AB. The final product (B) is:



Subtopic: Aromatic Hydrocarbons - Benzene - Structure, Preparation & Chemical Reactions |
Level 3: 35%-60%
Hints
In which form is the chlorine atom most feasibly liberated from the provided compound?

1.
2. Cl-
3. Cl
4. Cl2+
Subtopic: Aromatic Hydrocarbons - Benzene - Structure, Preparation & Chemical Reactions |
76%
Level 2: 60%+
Hints
The major product expected from the mono-bromination of phenyl benzoate is:
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Subtopic: Aromatic Hydrocarbons - Benzene - Structure, Preparation & Chemical Reactions |
Level 3: 35%-60%
Hints
Unlock IMPORTANT QUESTION
This question was bookmarked by 5 NEET 2025 toppers during their NEETprep journey. Get Target Batch to see this question.
✨ Perfect for quick revision & accuracy boost
Buy Target Batch
Access all premium questions instantly
(A) C8H10 (B) C8H6O4 C8H5BrO4 (C) (one-product only)
The structure of A in the above-mentioned reaction is-
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Aromatic Hydrocarbons - Benzene - Structure, Preparation & Chemical Reactions |
Level 3: 35%-60%
Hints
Unlock IMPORTANT QUESTION
This question was bookmarked by 5 NEET 2025 toppers during their NEETprep journey. Get Target Batch to see this question.
✨ Perfect for quick revision & accuracy boost
Buy Target Batch
Access all premium questions instantly
, unknown reagent (c) is:
1. LiALH4
2. NaBH4
3. LiAlH4(t-BuO)3
4. PCC/CH2CL2
Subtopic: Aromatic Hydrocarbons - Benzene - Structure, Preparation & Chemical Reactions |
51%
Level 3: 35%-60%
Hints
Unlock IMPORTANT QUESTION
This question was bookmarked by 5 NEET 2025 toppers during their NEETprep journey. Get Target Batch to see this question.
✨ Perfect for quick revision & accuracy boost
Buy Target Batch
Access all premium questions instantly

The product (B) in the above-mentioned reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Aromatic Hydrocarbons - Benzene - Structure, Preparation & Chemical Reactions |
52%
Level 3: 35%-60%
Hints

(a)
(b)
(c)
(d)
Subtopic: Aromatic Hydrocarbons - Benzene - Structure, Preparation & Chemical Reactions |
70%
Level 2: 60%+
Hints
Unlock IMPORTANT QUESTION
This question was bookmarked by 5 NEET 2025 toppers during their NEETprep journey. Get Target Batch to see this question.
✨ Perfect for quick revision & accuracy boost
Buy Target Batch
Access all premium questions instantly
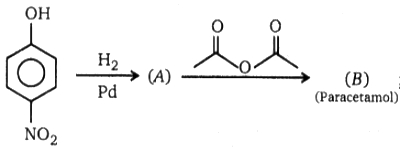 ; Product (B) of this reaction is :
; Product (B) of this reaction is :
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Aromatic Hydrocarbons - Benzene - Structure, Preparation & Chemical Reactions |
Level 3: 35%-60%
Hints
Cyclopentadiene is much more acidic than cyclopentane, because:
| 1. | Cyclopentadiene has conjugated double bonds. |
| 2. | Cyclopentadiene has both sp2 and sp3 hybridized carbon atoms. |
| 3. | Cyclopentadiene is a strain-free cyclic system. |
| 4. | Cyclopentadienyl anion ion, the conjugate base of cyclopentadiene, is an aromatic species and hence has higher stability. |
Subtopic: Aromatic Hydrocarbons - Benzene - Structure, Preparation & Chemical Reactions |
67%
Level 2: 60%+
Hints
The compounds among the following that can be generated by Friedel craft acylation is/are -
| I. |  |
| II. |  |
| III. |  |
| IV. |  |
| 1. | II, III and IV | 2. | I, III and IV |
| 3. | I and II | 4. | II and III |
Subtopic: Aromatic Hydrocarbons - Benzene - Structure, Preparation & Chemical Reactions |
75%
Level 2: 60%+
Hints










