Which of the following statements accurately describes the stability of allyl and propyl carbocations?
1. Allyl carbocation (CH2=CH-CH2+) is more stable than propyl carbocation.
2. Propyl carbocation is more stable than allyl carbocation.
3. Both are equally stable.
4. None of the above.
Sulphur trioxide act as:
1. An electrophile
2. A nucleophile
3. A homolytic agent
4. A base
The correct order of nucleophilicity among the following is:
(I)
(II)
(III) CN-
(IV)
1. I > II > III > IV
2. IV > III > II > I
3. II > III > I > IV
4. III > II > I > IV
The compound which gives the most stable carbocation on dehydration:
2.
3. CH3 - CH2 - CH2 - CH2OH
Allyl isocyanide has:
1. 9 σ, and 4π-bonds.
2. 8 σ, and 5π-bonds
3. 9 σ, and 3π-bonds,
4. 8 σ, and 3 π bonds
Which of the following acids has the smallest dissociation constant?
(1) CH3CHFCOOH
(2) FCH2CH2COOH
(3) BrCH2CH2COOH
(4) CH3CHBrCOOH
Which of the following resonating structures of 1-methoxy-1,3-butadiene is least stable?
1. \(\stackrel{\ominus}{\mathrm{C}} \mathrm{H}_2-\mathrm{CH}=\mathrm{CH}-\mathrm{CH}=\stackrel{\oplus}{\mathrm{O}}-\mathrm{CH}_3\)
2. \(C H_2=\mathrm{CH}_2-\stackrel{\ominus}{\mathrm{C}} \mathrm{H}-\mathrm{CH}=\stackrel{\oplus}{\mathrm{O}}-\mathrm{CH}_3\)
3. \(\stackrel{\ominus}{\mathrm{C}} \mathrm{H}_2-\stackrel{\oplus}{\mathrm{C}} \mathrm{H}-\mathrm{CH}=\mathrm{CH}-\mathrm{O}-\mathrm{CH}_3\)
4. \(\mathrm{CH}_2=\mathrm{CH}-\stackrel{\ominus}{\mathrm{C}} \mathrm{H}-\stackrel{\oplus}{\mathrm{C}} \mathrm{H}-\mathrm{O}-\mathrm{CH}_3\)
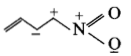
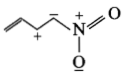
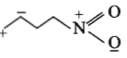
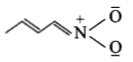
The least stable resonance structure among the following is:
1. 
2. 
3. 
4. 
Hyperconjugation involves the overlap of the following orbitals:
1. σ - σ
2. σ - p
3. p - p
4. π - π
The IUPAC name of the compound shown below is:
1. 2-bromo-6-chlorocyclohex-1-ene
2. 6-bromo-6-chlorocyclohexene
3. 3-bromo-1-chlorocyclohex-1-ene
4. 1-bromo-3-chlorocyclohexene








