In this reaction,
\(CH_{3}CHO \ + \ HCN \ \xrightarrow[]{} \ CH_{3}CH(OH)CN \ \xrightarrow[]{\textbf{H.OH}} \\ CH_{3}CH(OH)COOH\)
an asymmetric compound is generated. The acid obtained would be:
1. 50% D + 50% L-isomer
2. 20% D + 80% L-isomer
3. D-isomer
4. L-isomer

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the following has most acidic hydrogen?
1. 3-hexanone
2. 2,4-hexanedione
3. 2,5-hexanedione
4. 2,3-hexanedione
Acetaldehyde reacts with
1. Only electrophiles
2. Only nucleophiles
3. Only free radicals
4. Both electrophiles and nucleophiles

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which compounds will not reduce Fehling's solution?
1. Methanal
2. Ethanal
3. Trichloroethanal
4. Benzaldehyde

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Fehling's solution is:
1. Acidified copper sulphate solution
2. Ammoniacal cuprous chloride solution
3. Copper sulphate, Rochelle salt + NaOH
4. None of the above

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Benzaldehyde and acetaldehyde can be distinguished by:
1. Iodoform test
2. 2:4 DNP test
3. reaction
4. Wolff-Kishner's reduction

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The conversion of CH3OH to CH3COOH can be brought in by:
1. K2Cr2O7/H+
2. CO + Rh
3. KMnO4
4. H3PO4

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Polarisation of electrons in acrolein may be written as
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
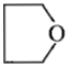
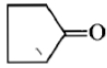
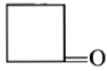
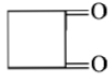
Dry distillation of barium salt of Hexane-1,2-dicarboxylic acid gives:
1. 
2. 
3. 
4. 
Ethyl ester P.
The product P will be
| 1. |  |
2. |  |
| 3. |  |
4. |  |






